Simple Visualisation of Cells on Alvetex® Scaffold Using Light Microscopy (Neutral Red-Methyl Blue Dye)
● Download this protocol as a PDF (1 MB)
1. Introduction
Cell culture in Alvetex Scaffold allows the formation of multilayered, high-density cell populations which approximate the complexity and structure of in vivo tissues. When viewing an unstained, unsectioned Alvetex Scaffold 3D culture under a standard brightfield microscope, the combined density and thickness of the scaffold and the 3D culture within it prevent the clear visualisation of individual cells. However, common visible dyes can be successfully used to confirm cell attachment or to check confluency.
Below are example protocols for visualisation of 3D cultures in Alvetex Scaffold using Methylene Blue (a destructive stain) and Neutral Red (a non-toxic stain). It is recommended to use a fresh Alvetex Scaffold disc for each time check, as the concentrations and/or nature of the dye used in the example protocol below can be either destructive of perturb the experiment. Imaging of the specimens will also need to be optimised according to the specifications of the microscope used.
2. Cell Staining Protocol
- At the desired time point, remove medium from the culture to be stained and wash once with PBS.
- Transfer the Alvetex Scaffold disc/well inserts to be stained to a fresh plate and return the remaining cultures to the 37 °C, 5 % CO2 cell culture incubator.
- Perform staining by the addition of Neutral Red solution (Sigma, N6264), or Methylene Blue solution (Sigma, 03978). Dilute latter 1:1 with PBS before use.
- Add carefully 500 µL of staining solution for cultures grown in 12 well plate formats (AVP002), whereas Alvetex Scaffold in 6 and 12 well insert formats (AVP004 and AVP005), will require 150 and 100 µL respectively.
- Leave the cultures in staining solution for 5 minutes at room temperature.
- Wash the cultures carefully and gently with PBS. For cultures grown in 12 well plate formats, perform 2 washes in PBS (4 mL/well), with gentle agitation (e.g. 100 rpm) for 5 minutes for each wash on a platform shaker.
- For cultures grown in multiwell insert formats, place the well insert into a Petri dish with copious amounts of PBS and perform a single wash with gentle agitation (e.g. 100 strokes/min on reciprocating shaker) for 5 minutes.
Note: Methylene Blue-stained cultures may require an additional washing step if the second wash is still very blue in appearance.
- After removing the last PBS wash, add 150 µL of PBS to each culture and proceed with visualisation.
- Depending on intended use, i.e. macroscopic whole disc confluency or microscopic individual cell morphology, visualisation can be performed in different ways:
- Cultures in their original holders can be viewed directly by eye or photographed. (See 3.0. Macroscopic visualisation of cells on Alvetex Scaffold).
- Cultures in their original holders can be checked under brightfield illumination on a conventional microscope compatible with tissue-culture plastic. (See 4.0. Microscopic visualisation of cells on Alvetex Scaffold).
- Cultures can be separated from their holders, placed on a microscope glass slide with a drop of PBS and checked under brightfield illumination on a conventional microscope compatible with glass coverslips. (See 4.0. Microscopic visualisation of cells on Alvetex Scaffold).
- As the method described above is intended for quick and convenient cell visualisation, the cells are not fixed. They must therefore be kept wet with a small amount of PBS and be examined immediately after staining.
3. Example results
3.1. Preparation of CHO-K1 Cultures on Alvetex Scaffold
CHO-K1 cells (ATCC, CCL-61) were seeded on Alvetex Scaffold 12 well plate format (AVP002) at a density of 0 cells, 0.5 million cells and 2 million cells per scaffold, in a seeding volume of 150 μL with a settling time of 30 minutes before adding 4 ml Kaighn’s modified nutrient mixture consisting of F-12K (Gibco, 21127022) supplemented with 10 % (v/v) foetal calf serum, 100 μg/mL penicillin and 10 μg/mL streptomycin. (See also Example protocols for the culture of the CHO-K1 cell line on Alvetex Scaffold). Cells were visualised using the Neutral Red staining technique after 1 day.
CHO-K1 cells were also seeded on Alvetex Scaffold 6 well plate format (AVP004) at a density of 0.5 million cells per scaffold as above and were maintained by complete media exchange every two days for 6 days. Cells were visualised using the Neutral Red staining technique after 2, 4 and 6 days.
3.2. Preparation of HepG2 Cultures on Alvetex Scaffold
HepG2 cells (ATCC, HB-8065) were seeded on Alvetex Scaffold 12 well plate format (AVP002) at a density of 0 cells, 0.5 million cells and 2 million cells per scaffold, in a seeding volume of 150 μL and with a settling time of 30 minutes before adding 4 mL of EMEM cell culture medium (Gibco, 21090055) supplemented with 10 % (v/v) foetal calf serum, 2 mM L-glutamine, 100 μg/mL Penicillin and 10 μg/mL Streptomycin. (See also Example protocols for the culture of the HepG2 cell line on Alvetex Scaffold). Cells were visualised using the Methylene Blue staining technique after 1 day.
3.3. Macroscopic Visualisation of Cells on Alvetex Scaffold
Immediately after staining, the gross appearance of the cultures were photographed (Figure 1.) Both Neutral Red and Methylene Blue are equally suitable for quick visualisation of cultures on Alvetex Scaffold discs.
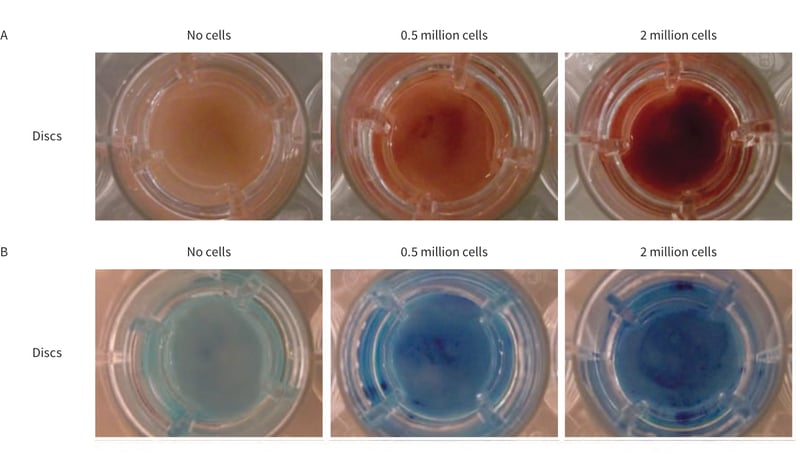
Figure 1. Macroscopic appearance of cultures on Alvetex Scaffold. A. Neutral Red staining of CHO-K1 cells after 24 hours. B. Methylene Blue staining of HepG2 cells after 24 hours. Note the increase in staining intensity with higher cell numbers.
3.4. Microscopic Visualisation of Cells on Alvetex Scaffold
REPROCELL recommends the use of Neutral Red staining for the visualisation of individual cells within the scaffold over Methylene Blue. Immediately after staining with Neutral Red, the microscopic appearance of the cultures was observed using a Leica ICC50HD microscope using LAS EZ software.
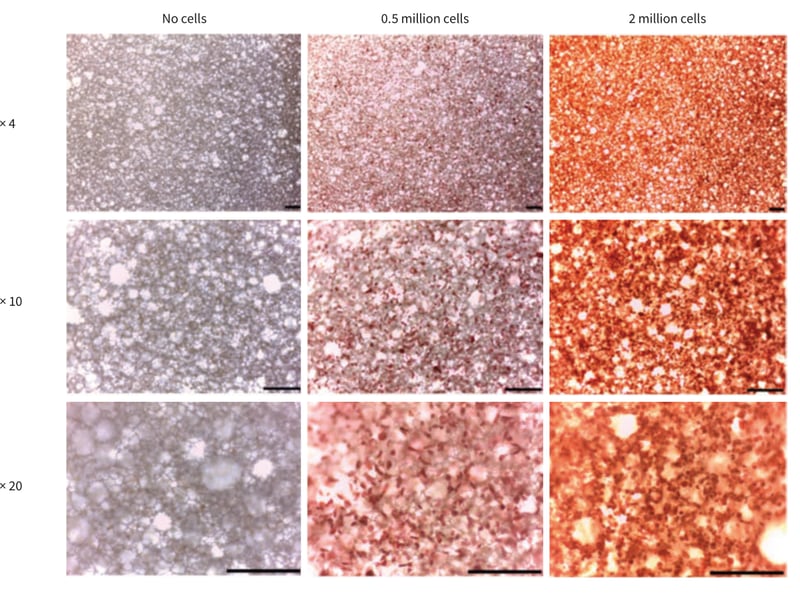 Figure 2. Microscopic appearance of CHO-K1 cells on Alvetex Scaffold after 24 hours as visualised by Neutral Red staining. Note the increase in staining intensity with higher cell numbers. Scale bar 200 µm.
Figure 2. Microscopic appearance of CHO-K1 cells on Alvetex Scaffold after 24 hours as visualised by Neutral Red staining. Note the increase in staining intensity with higher cell numbers. Scale bar 200 µm.
Neutral Red solution can be used as a very quick and simple staining technique to follow culture growth and cell survival within Alvetex Scaffold over a time course. Note as cell density increased with time, the staining intensity also increased both macroscopically and microscopically (Figures 3 and 4). Although Neutral Red can be compatible with live cell experiments as it is non-toxic at low concentrations, it is recommended that the user should check the effect of the dye on culture growth and cell survival first. REPROCELL recommends setting up extra scaffolds for dye analysis alongside with the experimental setup.
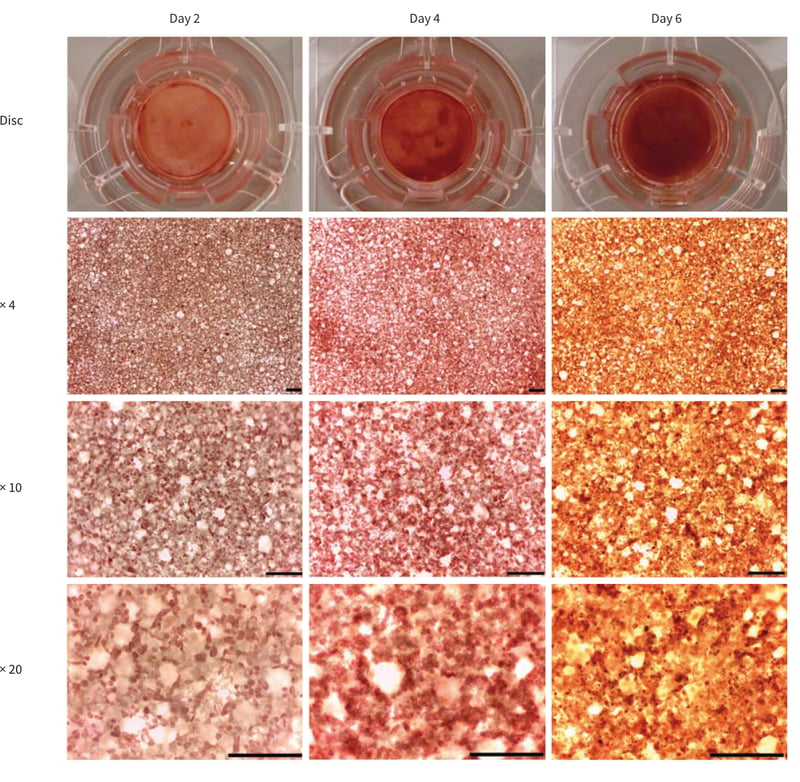 Figure 3. Neutral red staining of CHO-K1 cells cultured in Alvetex Scaffold 6 well inserts (AVP004) in 6 well plate format for up to 6 days. Cells were seeded at an initial seeding density of 0.5 × 106 cells/well. Note the intensity of the staining and the cell surface coverage increase with greater culture period. Scale bar 200 µm.
Figure 3. Neutral red staining of CHO-K1 cells cultured in Alvetex Scaffold 6 well inserts (AVP004) in 6 well plate format for up to 6 days. Cells were seeded at an initial seeding density of 0.5 × 106 cells/well. Note the intensity of the staining and the cell surface coverage increase with greater culture period. Scale bar 200 µm.
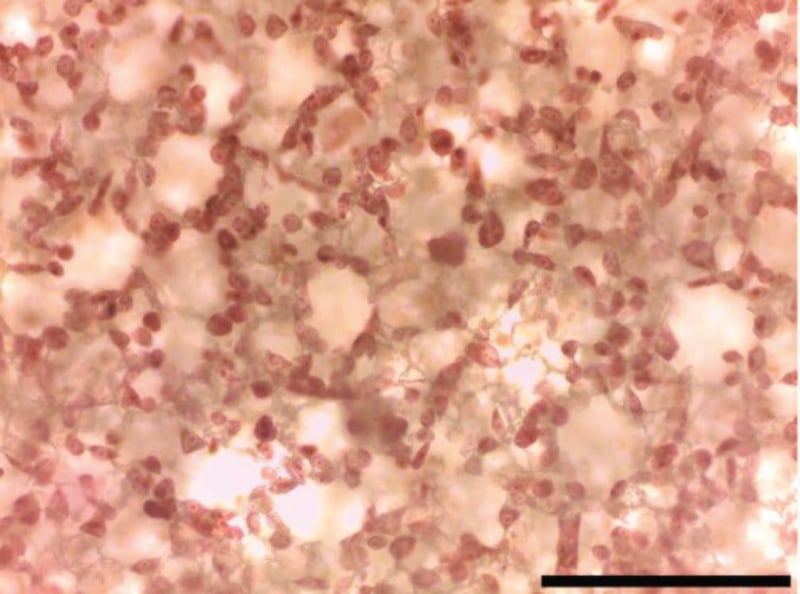
Figure 4. Close up view of Figure 3. Day 2 cells viewed by 20× objective. Note that this staining method clearly enables the visualisation of individual cell nuclear morphologies. Scale bar 200 microns.