Alvetex Scaffold Application Note 14
Target-based drug activity and post-translational and transcriptional analysis using ex vivo Alvetex Scaffold three-dimensional cell culture technology
Anne-Lise Peille[1], Susanne Braum[2], Richard Rowling[3], Torsten Giesemann[1], Vincent Vuaroqueaux[1], Heiner Fiebig[1], Stefan Przyborski[3], Sumeer Dhar[1]

Download this application note as a PDF (1.41 MB)
Introduction
Oncotest* maintains a unique repository of > 300 tumor xenograft models representing all major tumor histotypes (non-small cell lung cancer/NSCLC, pancreatic, prostate, colon, gastric, breast, ovarian and renal cancers, melanomas and sarcomas) as well as niche tumors (pleura-mesothelioma, bladder, and head and neck cancers). After evaluating many 3D cell culture platforms, Oncotest has chosen Alvetex Scaffold 96-well plates because of their simplicity, low cost of adoption and flexibility to study tumour cell cytotoxicity as well as mechanism of drug action at the translational and post-transcriptional levels.
*Oncotest GMBH was Acquired by Charles River Laboratories International Inc. in 2015.
Aims:
- Validation of the 3D tumour cell culture assay in Alvetex Scaffold to study the cell viability of tumor cells treated with targeted drugs.
- Comparison of the results obtained using the 3D cell culture assay in Alvetex Scaffold 96-well plates with TCA (soft agar) assay
- Demonstration of the possibilities for further analyses, such as Western blot, qRT-PCR and sequencing.
Materials and methods
Soft agar clonogenic assay
Xenografts growing s.c. in NMRI nu/nu mice were removed, mechanically disaggregated and treated with an enzyme cocktail to obtain a cell suspension. The percentage of viable cells was determined by trypan blue exclusion.
The clonogenic assay was performed according to the method described by Hamburger and Salmon. The test compounds were applied by continuous exposure in culture medium 24 h after seeding, using the three-fold concentrated stock solutions.
The assay was performed in triplicate at six concentrations, and incubated for 7 to 20 days. Colonies were counted with an image analysis system (OMNICON 3600, BioLogics, Inc. or Bioreader® 5000 Pro-W, Biosys GmbH) after staining with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride.
3D cell-based assay using 96-well Alvetex plates
Alvetex Scaffold is an inert, highly porous polystyrene scaffold for the 3D culture of cells. It allows cells to be cultured in a way that better mimics in vivo cell growth, offering improved cell viability and more physiologically relevant responses to drugs and other external stimuli. The Alvetex Scaffold 96-well plates were prepared and hydrated according to the instructions provided by REPROCELL. To enable direct comparison of the data obtained from the soft agar assay and Alvetex Scaffold plates, all conditions (seeding density, incubation time and compound treatment) were identical.
Drug and compound dispensing, and measurement of cell viability
The plates are incubated overnight at 7.5 % CO2 and 37 °C. Drug and compound preparation was performed using the TECAN Freedom EVO 200 automated platform. For the subsequent addition of compounds to each plate the MultiChannel Arm™ (MCA 96) was employed.
The plates were incubated for 8 or 13 days before being processed for the endpoint assay measurement. No medium change was performed during the incubation. At the end of the incubation cell viability was checked with CellTiter-Glo® (Promega) and luminescence was measured using a Perkin Elmer EnVision® multimode plate reader.
Obtaining RNA or DNA preparations with a sufficient quality for PCR, qPCR or sequencing is very difficult, if not impossible, with cells cultured in TCA and hydrogels. Alvetex Scaffold in contrast gives a new solution for molecular biology analyses since RNA and DNA extractions are very easy.
Quantitative PCR performed on non small cell lung cancer cells cultured in Alvetex Scaffold plates and treated with cMETi ± EGFRi were extracted and reverse transcribed with the SYBR® Green Cell-to-CT kit (Ambion). The cDNA obtained was used as a matrix to perform qPCR analyses of c-MET, EGFR, 18s and hTBP expression (data not shown). Analyses are also ongoing to demonstrate the possibility of this system being used to study mutations or gene amplifications/deletions.
Results
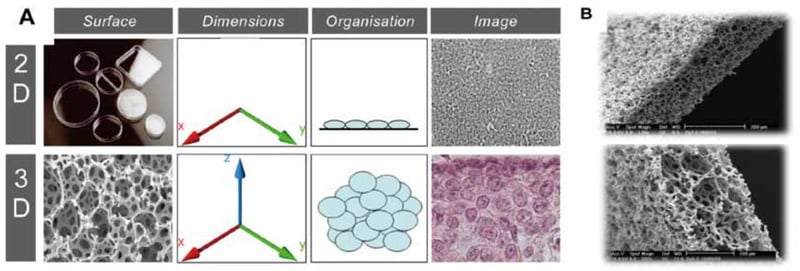
Figure 1. Alvetex Scaffold 3D cell culture technology. (A.) The Alvetex Scaffold is a 200 µm thick membrane with > 90 % porosity. Cells form in vivo like structures in the scaffold. (B.) Electron microscope images of the scaffold at different magnifications.

Figure 2. Freedom EVO 200 with 8-channel Liquid Handling Arm, MCA 96 and integrated gripper, cooling carrier, shaker, carousel and incubator (StoreX 110, LiCONiC).
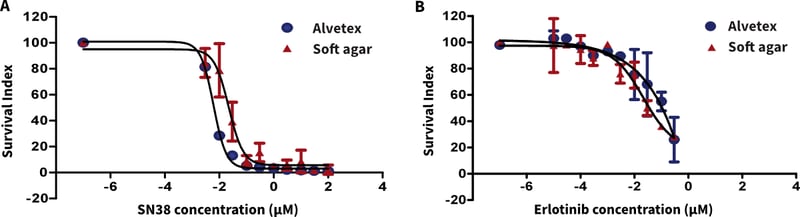
Figure 3. Comparison of Alvetex Scaffold vs soft agar clonogenic assay (TCA) for bladder cancer xenograft suspensions treated with (A.) SN38 (irinotecan) and (B.) erlotinib.
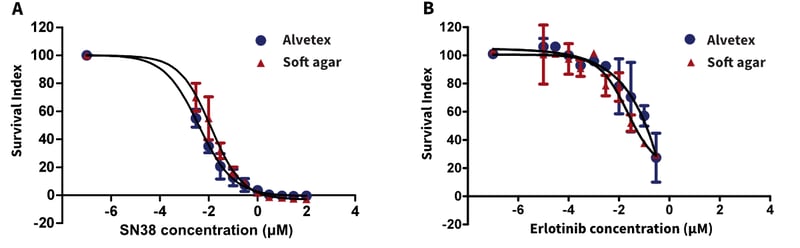
Figure 4. Comparison of Alvetex Scaffold vs soft agar clonogenic assay (TCA) for non-small cell lung cancer xenograft derived cell suspension treated with (A.) SN38 (irinotecan) and (B.) erlotinib.
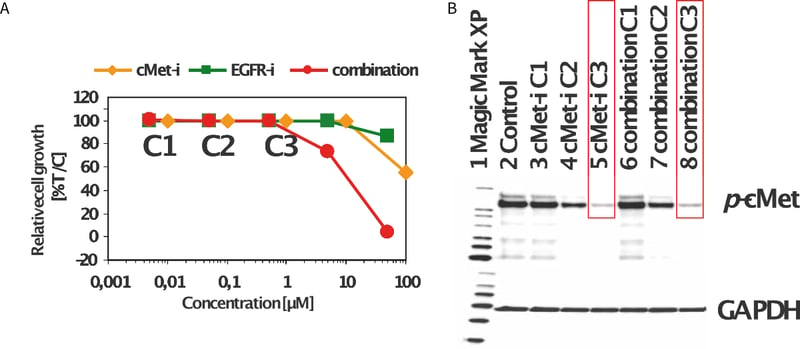
Figure 5. Non-small cell lung cancer xenograft cell suspensions were cultured on Alvetex Scaffold 96-well plates under standard 3D cell culture conditions and exposed to cMETi and EGFRi, alone and in combination. The viability was analyzed with Cell Titer-Glo® assay from Promega. The combination of cMETi and EGFRi enhanced the cell loss as compared to the single agent treatment (A). Whole cell lysates were prepared from the above cells for immunoblotting with phosphorylated c-Met antibody, as well as the GAPDH antibody as a loading control (B).
Summary and Conclusions
Alvetex Scaffold 96 well plates present a simple and reliable supplement to the clonogenic soft agar (TCA) assay for the screening of novel anti-cancer drugs.
The technology is compatible with common luminescent fluorescence cell health assays reading directly from the plates. In our hands, tumour cells can be cultured for 7-14 days without the need for media changes making the solution very cost effective.
The assay was easily automated on the TECAN Freedom EVO platform. The ability to reliably recover RNA and protein from the drug-treated 3D cells from Alvetex Scaffold offers effective analysis of drug mechanism of action using both protein and gene expression techniques.