Alvetex Scaffold Application Note 13
Three-Dimensional Culture of HaCaT Keratinocytes Using Alvetex Scaffold
Download this application note as a PDF (9.97 MB)
Highlights:
- HaCaT keratinocytes grown on Alvetex Scaffold recapitulate certain features of in vivo-like skin growth and organisation
- Cells undergo differentiation in a predictable manner and produce an orderly 3D structure
- Evidence shows the formation of the stratum corneum and production of cornified envelops
- Cultures of HaCaT cells can be maintained for several weeks
Key Benefits:
- Simple setup enables routine handling overcoming technical challenges
- Use of collagen gels simplified and improved
- Create 3D skin constructs to mimic aspects of epidermal barrier function
- Develop complex organotypic models including dermal equivalents
- Increase lifespan and value of skin construct
Skin's uppermost layer, the stratum corneum, provides its principal barrier of the skin. The stratum corneum consists of flat, dead keratinocytes (corneocytes) surrounded by a lipid bilayer matrix, which forms a dense and compact structure often described as “brick and mortar” [1]. The corneocytes are filled with keratin filaments, which are surrounded by a densely crosslinked protein layer, the cell envelope.
A lipid envelope is chemically linked to the densely packed cell envelope. This lipid monolayer provides the interface between the hydrophilic corneocytes and the lipophilic non-polar lipids which are surrounding the corneocytes. The corneocytes are interconnected by corneo-desmosomes which are important for stratum corneum cohesion. The change in lipid composition and cell structure during terminal differentiation produces a very densely packed structure in the stratum corneum. This impermeable nature of the cornified envelope is what helps direct the penetration of substances between the corneocytes [2].
The HaCaT lineage represents a spontaneously immortalised human keratinocyte cell line that still maintains some capacity for differentiation but generally lacks some elements of ordered structure and regular keratinisation [3,4]. HaCaTs have also shown drastically reduced tissue regeneration compared to normal epidermal keratinocytes [3,4]. Although this deficiency was not due to the permanent loss of their differential functions and can be rescued by the addition of growth factors such as transforming growth factor (TGF-ɑ). However, other reports in the literature have shown that the HaCaT cell line expresses a high degree of differentiation under organotypic conditions [5]. Schoop and colleagues (1999) declared the cell line as “an appropriate model for elucidation of the molecular mechanisms regulating keratinocyte growth and differentiation and for use in toxipharmacology.”
In this application note, we report the suitability of Alvetex Scaffold for keratinocyte culture using the HaCaT lineage. Initially, the scaffolds were tested as supports for fibroblasts and collagen matrices, to act as the dermal component of conventional organotypic cultures. Subsequently, however, it was found that keratinocyte stratification could also be achieved by growing the cells inside the scaffold at the air/ liquid interface, without any underlying collagen or fibroblasts. The second model shows considerable advantages over traditional organotypic approaches. The scaffold is completely synthetic and reproducible, removing inherent variability in the quality of dermal substrates. The process for creating the culture is also shortened and simplified as there is no need for collagen gel formation or de-epidermised dermis isolation.
Our results indicate that is possible to use Alvetex Scaffold to support the formation of a basic stratum corneum with or without any dermal equivalent. We propose that the described HaCaT model without collagen or fibroblasts is a useful tool to test epidermal barrier function. A future goal of this project is to establish the epidermal barrier using primary epidermal and dermal cells and test the barrier function by examining the permeability of various chemical compounds.
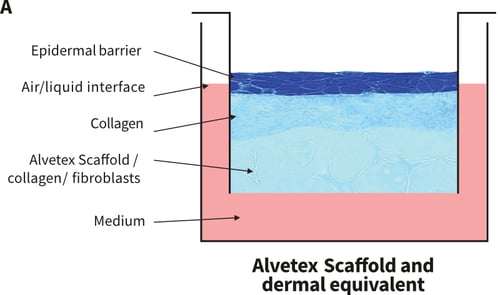
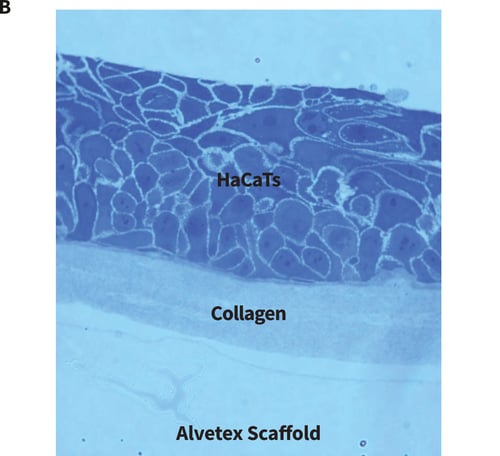
Figure 1. Organotypic co-culture and collagen gel set-ups. (A) Schematic diagram illustrating how HaCaT cultures can be set-up at the air-liquid interface in a well insert containing Alvetex Scaffold. There are several alternative permutations. For example, fibroblasts can be seeded into the scaffold prior to seeding keratinocytes on the surface. Alternatively, a collagen gel can be layered on the surface of the scaffold and keratinocytes seeded onto the surface of the gel. It is also feasible to use a combination of the gel and the fibroblasts. Collagen gels are classically used in RAFT cultures where keratinocytes are seeded onto the gel and brought to the air-liquid interface. However, such gels are notoriously difficult to work with and often shrink or tear. Working with a collagen gel layer on Alvetex Scaffold helps stabilise the gel by providing it with support during handling and reduces shrinkage. (B) Brightfield micrograph showing HaCaTs cultured on the surface of a collagen gel cast onto Alvetex Scaffold.
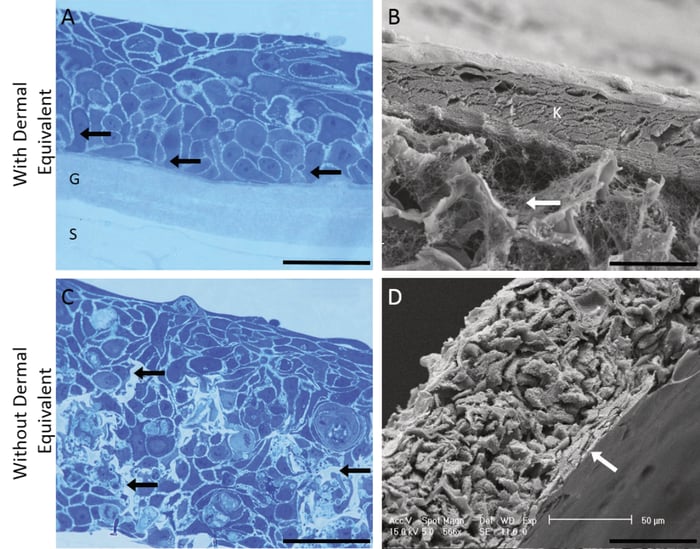
Figure 2. Growth of HaCaT keratinocytes on Alvetex Scaffold. The growth of HaCaT keratinocytes was evaluated on Alvetex Scaffold in the presence (A, B) and absence (C, D) of a collagen gel coating. Fibroblasts were initially cultured in Alvetex prior to seeding of HaCaT cells (A, B). 3D cultures were imaged using either bright field microscopy of resin sectioned, Toluidine Blue stained samples (A, C) or by scanning electron microscopy (SEM) (B, D). Image panels in detail: (A) Toluidine Blue stained L R White resin thin section (1 μm) of organotypic culture with 3D scaffold filled with collagen and fibroblasts cultured at the air/ liquid interface for 7 days. (Scale bar 100 μm). The scaffold (S) and layer of collagen gel (G) are labelled and black arrows indicate basal keratinocytes of columnar morphology; (B) SEM image of keratinocytes cultured for 7 days at air-liquid interface supported by 3D Alvetex Scaffold pre-filled with collagen and fibroblasts (Scale bar 50 μm). The layer of keratinocytes (K) is labelled and the white arrow indicates presence of the collagen filaments in the 3D polymer scaffold; (C) Toluidine blue stained L R White resin thin section (1 μm) of keratinocytes cultured within the 3D scaffold with no collagen or fibroblasts for 7 days (Scale bar 100 μm). The black arrow indicates the 3D scaffold; (D) SEM image showing keratinocytes cultured within the 3D scaffold with no collagen or fibroblasts at air/liquid interface for 7 days (Scale bar 50 μm). The upper surface of flattened stratified cells is indicated with a white arrow.
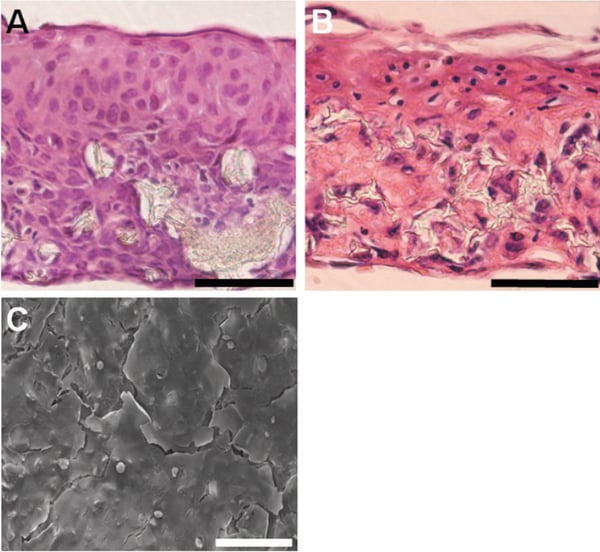
Figure 3. Flattening and stratification of HaCaT cells at the air-liquid interface. Imaging reveals that HaCaT keratinocytes stratify and flatten on the culture surface at the air-liquid interface. Images show brightfield micrographs of Haematoxylin and Eosin stained paraffin sections of HaCaT keratinocytes cultured within the scaffold with no collagen or fibroblasts and maintained at the liquid/air interface for 14 (A) or 35 (B) days. SEM imaging from above of the top surface of keratinocytes cultured at the liquid/air interface for 14 days (C). Evidence of stratification and the formation of flattened corneocytes lifting from the top surface of the epidermis can be clearly seen. Scale bars: 100 μm (A-C).
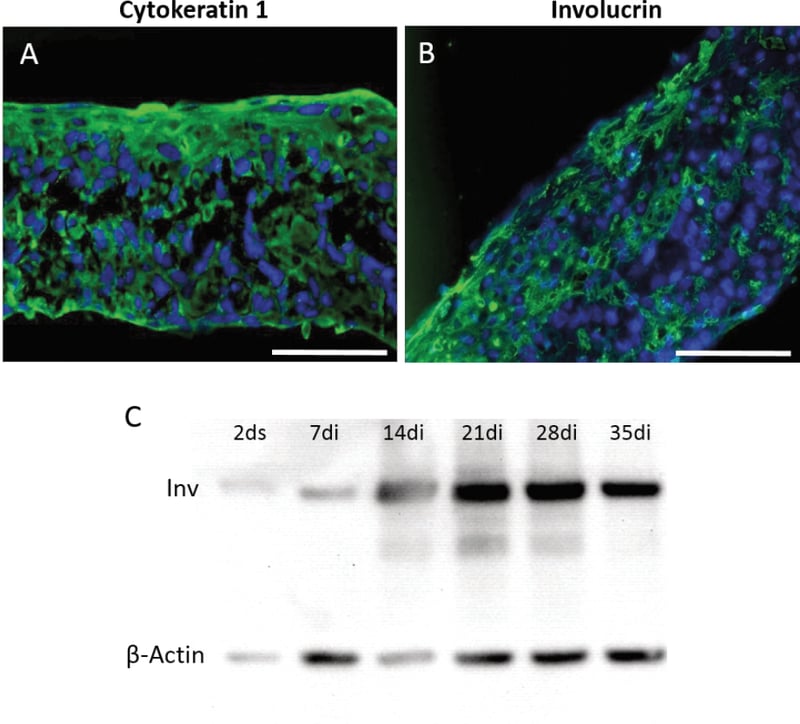
Figure 4. Characterisation of HaCaT keratinocytes on Alvetex Scaffold. Expression profiles of proteins associated with keratinocyte differentiation were assessed by immunocytochemistry (A, B) and western analysis (C). Keratin 1 (A) and Involucrin (B) expression after 35 days at air-liquid interface without collagen or fibroblasts; Western blot analysis showing Involucrin expression over time for cells cultured in the 3D scaffold without collagen or fibroblasts (ds: days submerged; di days at air/liquid interface). Scale bars: 100 μm (A, B).
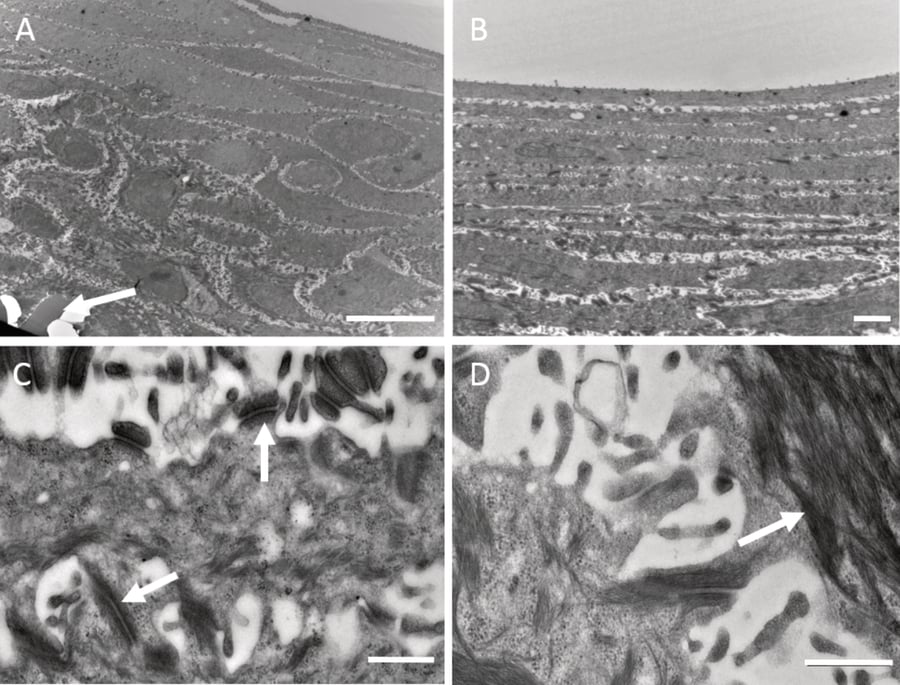
Figure 5. Ultra-structural characterisation of HaCaT cells on Alvetex Scaffold. Transmission electron microscopy was performed on HaCaT keratinocytes cultured at air-liquid interface without collagen or fibroblasts for 14 days (A-D). The data show how the cellular ultra-structure differentiates and changes as cells migrate toward the surface. An example showing the scaffold at the bottom of the culture is indicated by the arrow (A). Cells progressively flatten as they move towards the upper surface at air-liquid interface (B). Higher magnification images show the presence of desmosomes (C, arrows) and evidence of keratin filaments bundling beneath the plasma membrane (D, arrow). Scale bars: (A) 10 μm; (B) 2 μm; (C, D) 500 nm.
Figure 6. Flattening and stratification of HaCaT cells at the air-liquid interface. Imaging reveals that HaCaT keratinocytes stratify and flatten on the culture surface at the air-liquid interface. Images show brightfield micrographs of Haematoxylin and Eosin stained paraffin sections of HaCaT keratinocytes cultured within the scaffold with no collagen or fibroblasts and maintained at the liquid/air interface for 14 (A) or 35 (B) days. SEM imaging from above of the top surface of keratinocytes cultured at the liquid/air interface for 14 days (C). Evidence of stratification and the formation of flattened corneocytes lifting from the top surface of the epidermis can be clearly seen. Scale bars: 100 μm (A-C).
References
- Bouwstra JA, and Ponec M (2006). The skin barrier in healthy and diseased state. Biochimica Et Biophysica Acta-Biomembranes 1758, 2080-2095.
- Simonetti O, Hoogstraate AJ, Bialik W, Kempenaar JA, Schrijvers A, Bodde HE, and Ponec M (1995). Visualisation of diffusion pathways across the stratum corneum of native and in vitro re-constructed epidermis by confocal laser-scanning microscopy. Archives of Dermatological Research 287, 465-473.
- Boelsma E, Verhoeven MCH, and Ponec M (1999). Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). Journal of Investigative Dermatology 112, 489-498.
- Yamada KM, and Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell, 2007. 130(4): p. 601-10.
- Maas-Szabowski N, Starker A, and Fusenig NE (2003). Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-alpha. Journal of Cell Science 116, 2937-2948.
- Schoop VM, Mirancea N, and Fusenig NE (1999). Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. Journal of Investigative Dermatology 112, 343-353.