Alvetex Scaffold Application Note 11
Toxicity assessment using HepG2 liver cell lines in Alvetex Scaffold 96 well plate
Download this application note as a PDF (0.55 MB)
Drug metabolism is a process in which drugs are chemically altered by cells to more water soluble metabolites to allow elimination in urine or bile or by increasing access to drug excretory transporters [1]. A feature of hepatic metabolism pathways is the never ending array of drug substrates that are successfully metabolised by the liver. The liver is perfectly designed as a drug removal organ. Most xenobiotics enter the body by absorption through the gastrointestinal tract and then venous drainage via the portal vein to the liver. Along with the combination of drug-metabolising enzymes and drug transporters present in the intestinal mucosa, the liver provides an efficient barrier that prevents xenobiotics entering the bodyʼs circulation.
Phase 1 metabolism is the basic structural alteration of the drug molecule. Phase 2 metabolism involves conjugation of water soluble moieties attached to the drug, which plays a role in detoxification and facilitates excretion. P450 enzymes are the major catalysts of phase 1 metabolism in the liver. A subset of the P450 enzyme family, CYP1, 2 and 3 gene families account for 70-80 % of all phase 1 dependent metabolism of therapeutic drugs. Phase 2 metabolism involves transferases which catalyse the transfer of the hydrophilic group to the drug. One of the important functions of phase 2 enzymes is the detoxification of reactive metabolites from phase 1 drug metabolism.
Drug metabolism usually produces metabolites with lower pharmacological activity and toxicity than the parent drug, but this is not always the case as shown by examples such as irinotecan, a drug used for chemotherapy of colorectal cancer. Here metabolic activation by CYP3A4 results in a compound known as SN-38 which is the active pharmacological agent. The SN-38 is subsequently detoxified in phase 2 metabolism by conjugation with a-glucoronic acid. Drug metabolism is also a factor in dose response related hepatotoxicity. Paracetamol (acetaminophen, APAP) is a well known example of dose related toxicity. P450 mediated phase 1 metabolism of paracetamol gives rise to the reactive intermediate N-acetyl-p-benzoquinone (NAPQI), which is detoxified by phase 2 conjugation with hepatic glutathione. In the event of paracetamol overdose the formation of NAPQI exhausts the glutathione stores and hepatic toxicity proceeds.
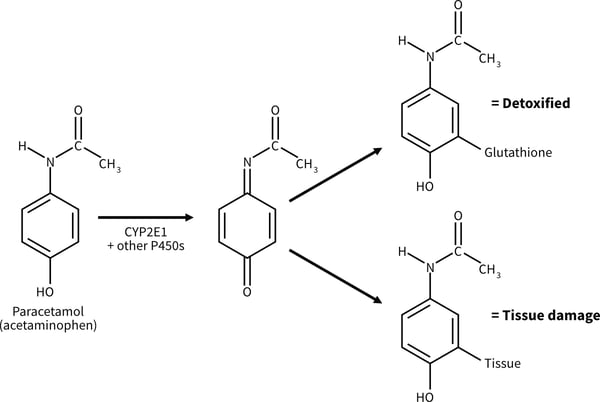
Figure 1. Metabolic activation of paracetamol (acetaminophen) to a hepatotoxic metabolite [1].
This application note describes the dose response of the HepG2 cell line to increasing concentrations of paracetamol screened on Alvetex Scaffold 96-well plate technology. The HepG2 cell line is one of the most widely used for evaluating the toxicity of chemicals and drugs [2]. HepG2 cells are conventionally cultured as a monolayer in a two-dimensional (2D) plate, but when grown in monolayer culture they express lower levels of the cytochrome P450 (CYP) enzymes, compared to primary human hepatocytes [3].
Method
HepG2 cells (100,000 cells/well) were cultured for up to 48 hours before exposure to paracetamol dissolved in culture medium (Acetaminophen, Sigma A7085) for up to 48 hours (1-50 mM). Viability was assessed using the MTT assay (see Section 4.1 of Alvetex Scaffold 96-well plate: Instructions for Use and Sample Applications) and percentage viability was determined by comparing to a non paracetamol treated control (designated 100% viability).
HepG2 cells showed a cytotoxic response to increasing dose concentrations of paracetamol. The responses differed between 3D and 2D cultures. In both Alvetex and 2D, an initial cytotoxic response was observed at between 10-20 mM paracetamol. After 24 hours exposure a higher percentage of HepG2 cells survive on Alvetex Scaffold above 10 mM concentration of paracetamol. After 48 hours paracetamol exposure, good dose response curves are observed on both 2D and Alvetex Scaffold plates with a higher resistance/cell survival observed at all paracetamol concentrations on Alvetex Scaffold leading to a higher IC50 value obtained on Alvetex Scaffold compared with 2D cultures (15.7 mM, and 11.9 mM respectively). 100 % cell death was observed at 20 mM and 30 mM paracetamol for 2D and Alvetex Scaffold format respectively.
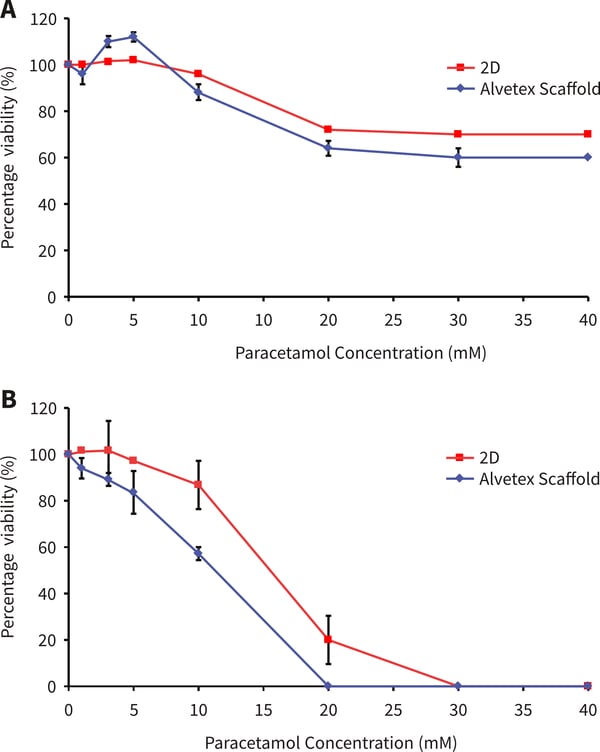
Figure. 2. Paracetamol dose response of HepG2 cells on Alvetex Scaffold 96 well plate technology. (A) 24 hours cell growth and 24 hours paracetamol exposure (n=3, ±SE); (B) 48 hours cell growth and 48 hours paracetamol exposure (n = 2, ± SE). Response A shows a similar pattern as published previously by Schutte et al. [4].
Conclusions
Alvetex Scaffold 96 well plate technology is a valuable tool for in vitro toxicological and pharmacological studies on HepG2 cells. This initial study using the HepG2 cell line is a useful guide for primary hepatocyte growth and toxicological studies on Alvetex Scaffold 96-well plate technology.
References
- The Textbook of Hepatology: From Basic Science to Clinical Practice, Section 2 Function of the Liver, 3rd edition, 2007.
- Knasmuller S, Mersch-Sundermann V, Kevekordes S, Darroudi F, Huber WW, Hoelzl C, Bichler J, and Majer BJ. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge, Toxicology, 198, 315–328 (2004)
- Wilkening S, Stahl F, and Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties, Drug Metab Dispos. 31, 1035–1042 (2003).
- Schutte M, Fox B, Baradez M, Devonshire A, Minguez J, Bokhari M, Przyborski S, and Marshall D. Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three-dimensional culture on a polystyrene scaffold designed for routine use. Assay Drug Dev Techn. 9, 475-486 (2011).