Alvetex Scaffold Application Note 07
Modelling the Growth of Simple Epithelial Monolayers Using Alvetex Scaffold
Download this application note as a PDF (13.21 MB)
Abstract
Despite the long-standing use of filter membranes and thick collagen matrices in the recreation of simple epithelial and mucosal 3D models in vitro, these substrates still present shortcomings in closely replicating the complexity of cell-cell and cell-matrix interactions found in vivo. Alvetex Scaffold is an inert, 200μm thick, highly porous scaffold which is suitable for the 3D culture of cell types originating from morphologically different epithelial tissues. The technology is also suitable for culture models of increasing complexity, from an even ECM-supported monolayer to a thick but porous mucosal co-culture.
Introduction
Mucosal tissue is found at the boundary between the body and the external environment. Its primary function as a site for solute exchange (e.g. squamous epithelium of the airways, columnar epithelium of the gut) also exposes it to exogenous agents and aggression which must be prevented from invading and affecting the underlying tissues. The epithelial cells that are found on the outermost layer of the mucosa present different morphologies (cuboidal, squamous or columnar) in accordance with their specific function. Their apical membrane can be further characterised by the presence of microvilli which increase their absorptive surface or cilia which drive localised currents and facilitate transport along the mucosa. Mucus-producing cells can be interspersed amongst the monolayer and secrete a protective mucus layer. The epithelial cells are separated from the other main constituent of the mucosa, the lamina propria, by a thin basement membrane. The lamina propria is loose connective tissue, which, in addition to capillaries, arterioles and fibroblasts, contains lymphocytes in readiness for any breach by exogenous material. Aside from mucosal tissues, simple epithelia are also found in linings inside the body such as blood vessels.
In vitro models of isolated simple epithelia are useful to detect intracellular events in response to external stimuli. However, if restricted to traditional 2D cell culture at the bottom of plastic dishes, they are incapable of replicating the principal function of simple epithelia, i.e. transport of agents, from small molecules to immune cells, across the epithelial monolayer. Filter membranes presented in well inserts, such as Transwell® and ThinCert®, have long been adopted throughout both industry and academia to assess cross-epithelial transport (eg. intestinal drug absorption [Balimane et al. 2006], epithelial transmigration by immune cells [Zemans et al. 2011]), but their ease-of-use has led to one important factor being overlooked, namely the role of the native basement membrane as a barrier to transport and migration across simple epithelia [Huber and Weiss, 1989]. As filter membranes, much like plastic dishes, allow the growth of confluent monolayers without pre-coating with ECM, the purposeful inclusion of this physiologically important layer is absent from the great majority of culture models involving filter membranes models. By contrast, Alvetex Scaffold surface topography precludes the formation of an even surface monolayer without pre-coating, and the introduction of a thin layer of ECM, e.g. collagen I, is a pre-requisite to recreate a flat growth surface, thus rectifying this all-too common physiologically-important omission. Alvetex Scaffold is 200 μm thick and highly porous, which ensures that solute transport is not compromised and even thin collagen gels can be supported and confidently handled.
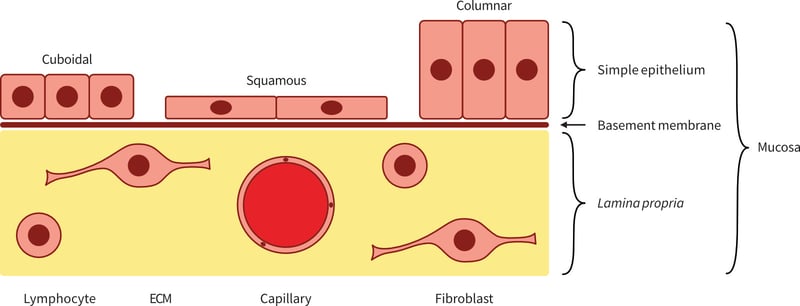
Figure 1: Schematic representation of mucosal tissue and its cellular constituents.
However useful mono-cultures of epithelial have been and continue to be in research and industry, it cannot be ignored that cross-talk between the cells of the epithelium and the fibroblasts within the lamina propria is involved in a number of biological processes, ranging from normal development (uro-genital tract, breast [Cunha et al.]) to wound healing (lung [Horowitz et al. 2006]), as well as cancer (breast [Parrinello et al. 2006], colon [Bosman et al. 1993] and prostate [Niu and Xia, 2009]).
Efforts to replicate and elucidate the in vivo interactions between the different cell types found in mucosal tissues within the confines of in vitro research have led to the development of several co-culture models. Two-dimensional (2D) mixed cultures of epithelial and lamina propria cells do allow cell-cell contact and signalling, but lack the correct tissue architecture found in mucosae in vivo, and do not easily lend themselves to an assessment of the presence or importance of cell migration during cells’ response to stimuli [Duell et al. 2011]. To allow co-culture of monolayers of different cell types, filter membranes presented in well inserts, such as Transwell® and ThinCert®, can be used. These set-ups are useful for determining the role of soluble factors in cell-cell signalling, applying different media to the apical and basal side of cells and, depending on pore size, helping to quantify short-distance migration from one side of the membrane to the other. However, they still rely on the use of monolayers, i.e. they lack the extensive cell-cell inter-actions and endogenous ECM production necessary to some aspects of cell behaviour, e.g. intestinal macro-phage differentiation [Spoettl et al. 2007]. To approximate the tissue thickness and cell-ECM interactions found in vivo, matrice-layered membranes can be used, whereby cells commonly found in the lamina propria are grown embedded in a thick matrix, with an epithelial monolayer seeded on top [Leonard et al. 2010]. This set-up most closely resembles the in vivo tissue architecture and allows the monitoring of cell migration through a thick and dense matrix environment, i.e. a very definite cell response requiring sustained proteolytic and motile cell activity, as opposed to short-distance movement between two sides of a thin membrane. The use of a default exogenous ECM, such as collagen or Matrigel®, does however present several disadvantages. Firstly, it provides an environment which is chemically distinct from the complex mix of ECM molecules that the cells are exposed to in vivo, and thus might introduce artefacts in cell behaviour. Secondly, the presence of exogenous ECM makes it harder to detect genuine endogenous ECM protein production from the cultured cells. Thirdly, the thickness of matrices generally produced to allow convenient handling does impede diffusion of solutes, creating a gradient of nutrients, signalling molecules and toxins, which induces heterogeneity of cell responses at different depths of the matrix [Raghavan et al. 2010].
Alvetex Scaffold is engineered from polystyrene to provide a consistent and inert highly porous structure for routine 3D cell culture. Its combined depth and porosity allows true 3D multilayer mono-culture or co-culture with minimal compromising of solute transport. Its inert chemical nature allows for the clear detection of cell- secreted ECM proteins or customised pre-coating with a range of ECM agents. Layering the top surface of Alvetex Scaffold with a thin layer of collagen readily produces a smooth surface for the growth of epithelial monolayers and can be used as such or combined with fibroblasts growing in the thickness of Alvetex Scaffold for more complex mucosal co-culture models.
In this application note, we demonstrate the versatility of Alvetex Scaffold in enabling culture of simple epithelia. As an example we use the human colon carcinoma cell line CaCo-2 to illustrate epithelial mono-culture as a collagen-supported confluent monolayer showing clear signs of enterocytic differentiation. In addition, we have develop-ed a mucosal co-culture model in combination with fibroblasts. We also use the kidney epithelial cell line MDCK to illustrate how the void structure characteristic of Alvetex Scaffold favours 3D epithelial mono-culture within the depth of the scaffold.
Highlights
- Coating the top surface of Alvetex Scaffold with an even collagen layer
- Differentiation of the intestinal epithelium in preparation for functional assays
- Establishment of co-culture for the study mucosal in vitro models
- Differentiation of densely-packed renal cyst-like structures in 3D
Key Benefits
- Creation of an epithelial monolayer at the surface or in the depth of 3D
- Inclusion of a thin layer ECM for absorption/ transmigration assays
- Minimal compromise on solute transport
- Use of a single platform to build increasingly complex models
Collagen I Coating of Alvetex Scaffold Provides ECM Support for Culture of Simple Epithelia
Alvetex Scaffold is a porous 3D scaffold which is highly conducive to the multi-layering of cells cultured in vitro. A large number of epithelia, however, naturally grow as a monolayer on the curved 2D surface of a basement membrane comprising of extracellular matrix (ECM) with underlying connective tissue. Coating the surface of Alvetex Scaffold with a layer of Collagen I creates a 2D ECM surface attachment for epithelial monolayers while allowing access to both the apical and basal sides of the cells. This set-up can also be further developed into mucosal models, as detailed later in this application note.
Applying a solution of 2 mg/mL Collagen I to Alvetex Scaffold results in coverage of the whole area of the scaffold (Figure 2A and 2B). The collagen layer is smooth and of even thickness (Figure 2C and 2D). When colo-rectal adenocarcinoma Caco-2 cells or kidney epithelial MDCK cells are cultured on collagen-coated Alvetex Scaffold, they readily organise into an epithelial mono-layer and do not invade into the collagen or the underlying 3D scaffold (Figure 3A and 3C). By contrast, both Caco-2 cells and MDCK cells cultured on uncoated Alvetex Scaffold readily penetrate into the scaffold (Figure 3B and 3D). Caco-2 cells multilayer in uncoated Alvetex Scaffold, as can be expected of any carcinoma cell line, while MDCK cells line the voids of uncoated Alvetex Scaffold to form structure reminiscent of renal tubules. This demonstrates the role of the ECM and environmental architecture in the determination of cell behaviour, as well as the versatility of Alvetex Scaffold and its suitability for a range of cell types.
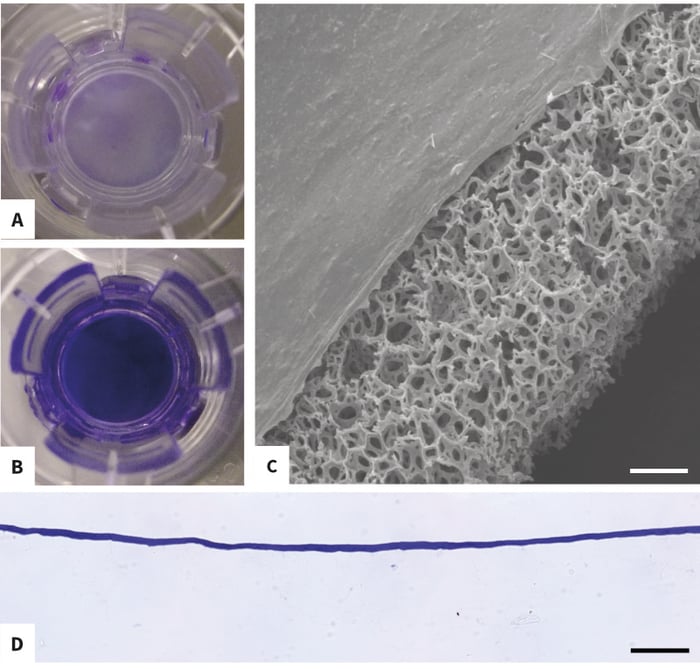
Figure 2: Morphology, Thickness and Coverage of Collagen I surface coating of Alvetex Scaffold. Coomassie Brilliant Blue R staining of discs presented in insert format demonstrates that Collagen I gels over the entire surface of the disc (A: untreated control, B: coated). Scanning electron microscopy (C) and wax-embedded sections (D) show that the collagen layer is even and of consistent thickness. Scale bars: C: 70 μm, D: 100 μm. (see protocols for detailed collagen coating protocol.)
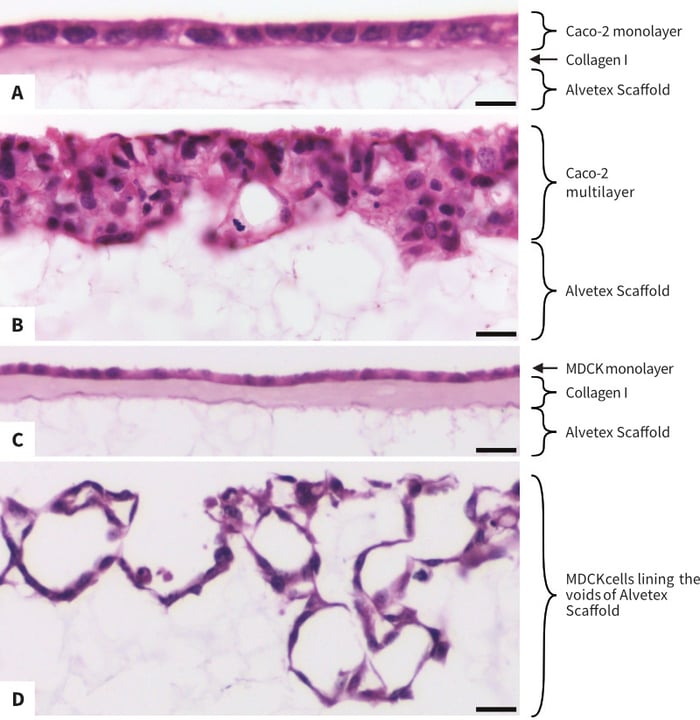
Figure 3: The morphology of cell populations in Alvetex Scaffold is controlled by Collagen I coating. High magnification brightfield micrographs show that both Caco-2 cells and MDCK cells cultured with Collagen I form an even epithelial monolayer (A, C), while the same cells cultured without Collagen I either readily invade Alvetex Scaffold and form a multilayer (Caco-2 cells, B) or line the surface of Alvetex Scaffold voids (MDCK, D). Caco-2 cells and MDCK cells were cultured for 7 days on 22mm diameter Alvetex Scaffold discs presented in 6-well insert in 6-well plate format. Cells were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin. Scale bars: A: 10 μm, B: 25 μm and C, D: 20 μm.
Differentiation of Caco-2 Cells into a Polarised Intestinal Epithelium of Alvetex Scaffold
Although Caco-2 cells have been used to model colon cancer cytotoxicity [Virag et al.], they are better known for their ability to differentiate into functional enterocytes in vitro, and are characterised by the presence of tight junctions, a brush border and brush-border-associated hydrolases [Chantret et al.]. This has led to their wide-spread use as in vitro models of intestinal drug absorption [Balimane et al.] and bacterial or viral gut infection [Gaillard et al, Svensson et al.].
10 days show evidence of brush border formation, as seen in resin-embedded Toluidine Blue-stained sections (Figure 4A), and confocal immunofluorescence of phalloidin-stained wholemounts (Figure 4B and 4C). In side view, phalloidin is strongly localised at the apical side of the monolayer (Figure 4B), where actin-rich microvilli are known to form the brush border characteristic of enterocytes. A Z-section of the apical end of the same region (Figure 4C) show clearly-defined cell borders, as well as punctuated staining of varying density consistent with cells at different stages of brush border formation. After 14 days’ culture, transmission electron microscopy (TEM) of the apical side of the monolayer show a dense brush border made up of evenly-sized microvilli (Figure 4D).
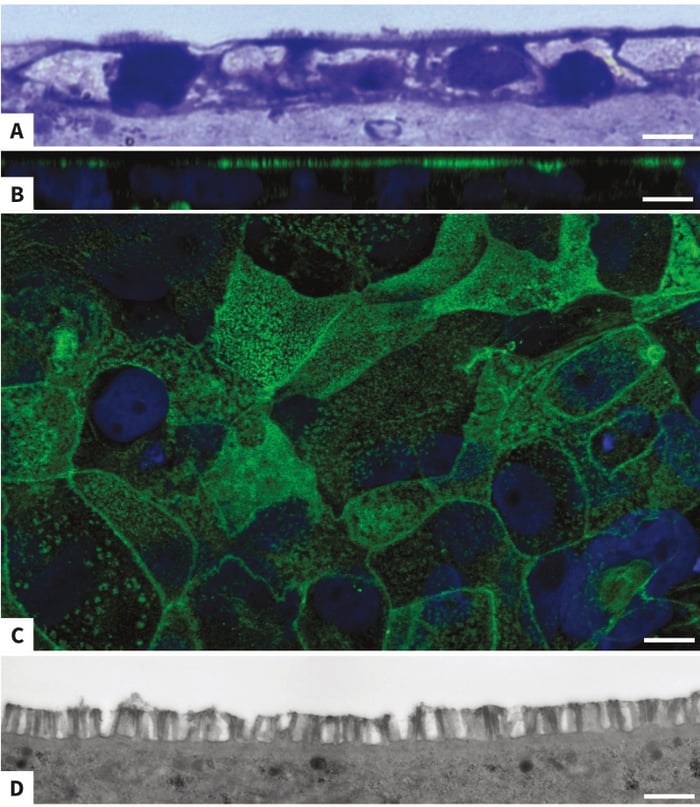
Figure 4: Brush Border formation in Caco-2 monolayer grown on collagen-coated Alvetex Scaffold. Toluidine-Blue staining of resin-embedded sections of Caco-2 cells grown on collagen-layered Alvetex Scaffold in insert format for 10 days show an apical brush border with microvilli typical of enterocyte differ-entiation (A: top view, apical end). Confocal microscopy with phalloidin reveals actin-rich microvilli at the apical region of 10 days-old cultures of Caco-2 cells (B: side view). Within the monolayer, cells at different stages of brush border formation, as well as lateral cell borders, are clearly visible (C: apical view). In 14 days-old cultures, transmission electron mic-roscopy (TEM) shows even-sized microvilli forming a dense brush border at the cells’ apical surface. Scale bars: 5 μm (A), 10 μm (B), 10 μm (C), 1 μm (D).
Confocal immunofluorescence of Caco-2 cells whole-mounts stained for the tight junction protein occludin reflects the typical cobblestone arrangement of cells within an epithelial monolayer, with stronger staining occurring at the vertices (Figure 5A). In side view, Occludin staining is clearly seen to be located at the apical side of the monolayer (Figure 5B). After 14 days’ culture, apical desmosomes are detected by TEM (Figure 5C).
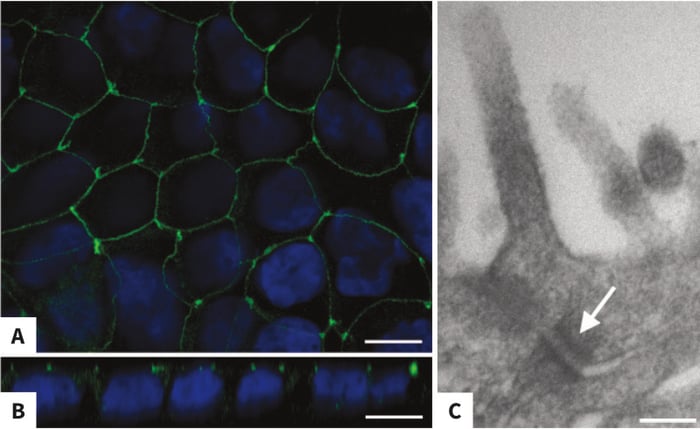
Figure 5: Apical Tight Junctions in Caco-2 monolayer grown on collagen-coated Alvetex Scaffold. Confocal microscopy with an antibody against occludin highlights the typical epithelial cobblestone arrangement of Caco-2 cells grown on collagen-layered Alvetex Scaffold in insert format for 10 days (A: top view). In side view, the apical localisation of Occludin within the layer is evident (B: side view). In 14 days-old cultures, Transmission Electron microscopy (TEM) reveals the presence of desmosomes near the cells’ apical surface (C: arrow). Scale bars: 10μm (A), 10μm (B), 130nm (C).
Taken together, these data demonstrate that Caco-2 cells cultured on collagen-coated Alvetex Scaffold readily differentiate into functional enterocytes in vitro, allowing their use in downstream applications such as intestinal models of drug absorption or infection.
Caco-2 Cell Co-culture with Colon Fibroblasts in Alvetex Scaffold
Co-culture of colon epithelial cells and colon fibroblasts to mimic the in vivo intestinal mucosa is an informative model to study epithelial-fibroblast interactions both during the proliferation, differentiation and repair of normal intestinal epithelium [Visco et al, Raymond et al.], as well as during pathological intestinal fibrosis related to gut inflammation or the presence of cancerous epithelial cells [Willemsen et al, Koshida et al.].
The layering of Alvetex Scaffold with collagen I immediately creates two separate compartments for co-culture, a smooth top surface for epithelial cells and a thick highly porous underlying volume which, when modelling intestinal mucosa, can accommodate a fibroblast population. In Figure 6, we show that the normal human colon fibroblast cell line CCD-18Co can be established in Alvetex Scaffold prior to layering with collagen I and addition of Caco-2 cells, with the resulting co-culture showing fibroblast cells in close association with the ECM and the retention of correct epithelial and fibroblastic morphology in both cell lines used, i.e. an even upper epithelial monolayer and well-spread underlying fibroblasts.
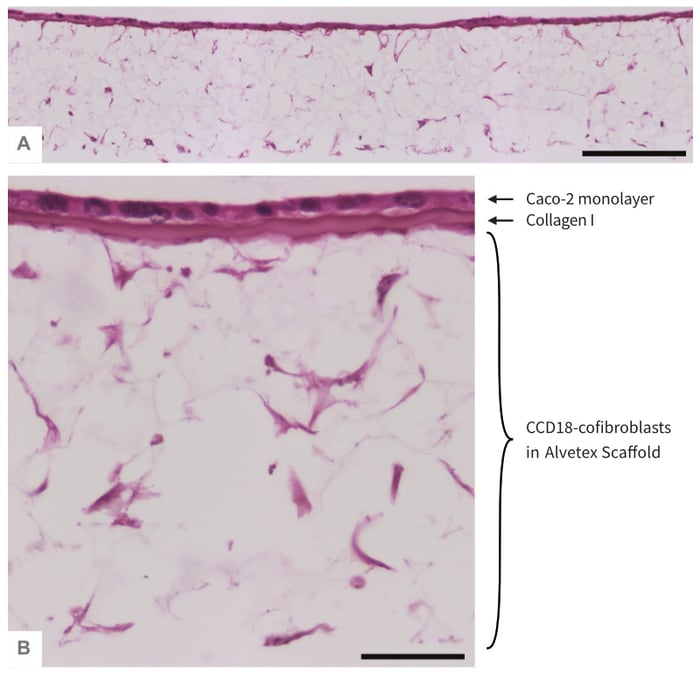 Figure 6: Co-culture ofCaco-2 cells and CCD-18 fibroblasts separated by a Collagen I layer in Alvetex Scaffold. Low (A) and high (B) magni-fication brightfield micro-graphs of an even monolayer of Caco-2 cells form at the collagen-coated top surface of Alvetex Scaffold, with CCD-18Co fibroblasts underneath the same collagen layer and distributed throughout the depth of Alvetex Scaffold. CCD-18Co fibroblasts were grown on 22 mm diameter Alvetex Scaffold discs presented in 6-well insert in 6-well plate format for 14 days prior to layering with Collagen I and seeding of Caco-2 cells. Co-cultures were grown for a further 5 days, after which they were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin. Scale bars: 200 μm (A) and 50 μm (B).
Figure 6: Co-culture ofCaco-2 cells and CCD-18 fibroblasts separated by a Collagen I layer in Alvetex Scaffold. Low (A) and high (B) magni-fication brightfield micro-graphs of an even monolayer of Caco-2 cells form at the collagen-coated top surface of Alvetex Scaffold, with CCD-18Co fibroblasts underneath the same collagen layer and distributed throughout the depth of Alvetex Scaffold. CCD-18Co fibroblasts were grown on 22 mm diameter Alvetex Scaffold discs presented in 6-well insert in 6-well plate format for 14 days prior to layering with Collagen I and seeding of Caco-2 cells. Co-cultures were grown for a further 5 days, after which they were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin. Scale bars: 200 μm (A) and 50 μm (B).
MDCK Cell Formation of Tubule-like Structures in Alvetex Scaffold
In vivo kidney tubules are lined with a monolayer of highly polarised epithelial cells. Several in vitro models are currently available to study kidney cell polarisation and to approximate tubulogenesis via the formation of tubules [Zuk et al, Wang et al, Raghavan et al.]. The growth of cell monolayers on Transwell® is convenient but lacks the 3-dimensionality of tubules, while tubule formation either requires pre-adaptation of cells to suspension culture or relies on embedding cells in a collagen matrix which slows down the diffusion of nutrients and waste products.
Alvetex Scaffold is a highly porous scaffold, consisting of voids with an average diameter of 40μm and interconnects with an approximately diameter of 13μm. When grown in Alvetex Scaffold, MDCK cells readily invade the scaffold and line the surface of the voids, thus recreating cyst-like structures. Importantly, these structures are adjacent rather than isolated, which more closely mimic in vivo tubules (Figure 7A vs 7B). Tight junctions and desmosomes are also formed at the apical regions of neighbouring cells growing within the depth of Alvetex Scaffold (Figure 7C and 7D).
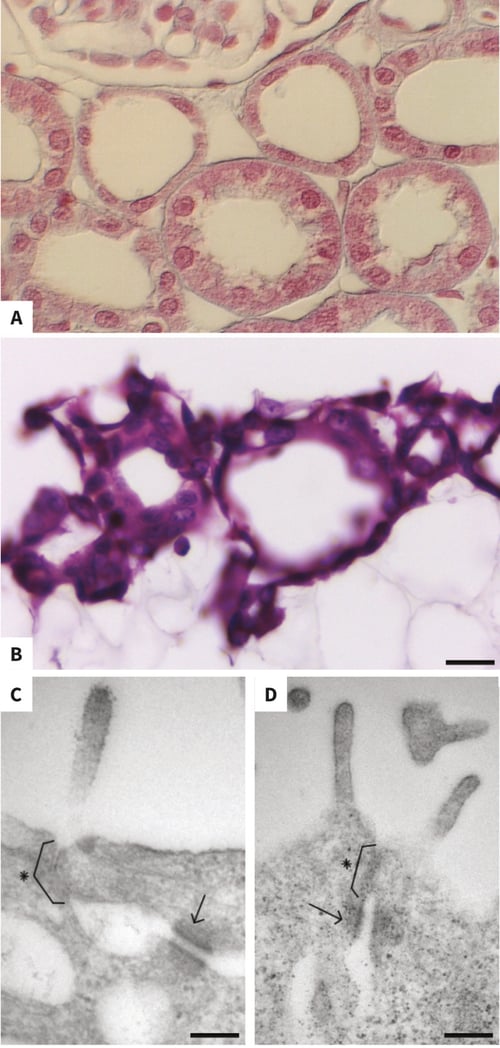
Figure 7: The morphology of MDCK cells in uncoated Alvetex Scaffold resembles in vivo kidney tubular structures. High magnification brightfield micrograph of in vivo kidney shows the typical morphology of both proximal and distal tubules (A, micrograph from www.cytochemistry.net). MDCK cells cultured for 7 days on 22 mm diameter Alvetex Scaffold discs presented in 6-well insert in 6-well plate format penetrate the scaffold and line the surface of the voids (B), forming structures reminiscent of those observed in vivo. After 10 days’ culture, tight junctions (brackets with asterixes) and desmosomes (arrows) have formed between the apical regions of neighbouring MDCK cells established within the depth of Alvetex Scaffold (C, D). Note that microvili are also present. Cells were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin (B) or were fixed Karnovsky’s buffer, post-fixed in osmium tetroxide, embedded in LR white resin, sectioned (70 μm) and viewed by TEM (C, D). Scale bars: 20 μm (B), 150 nm (C), 200 nm (D).
When MDCK cells are cultured on top of collagen-layered Alvetex Scaffold, they form a confluent monolayer of epithelial morphology after 10 days’ culture, as shown by confocal microscopy of phalloidin-stained unsectioned wholemounts (Figure 8A). The confluency of the mono-layer is confirmed in sideview, with lateral phalloidin staining and apical occluding staining between neighbouring cells (Figure 8B and 8C) TEM imaging of sectioned cultures clearly shows that cells are cuboidal and possess apical microvili (Figure 8D and 8E), with cell-cell junctions including both tight junctions and desmosomes in the apical region (Figure 8E) and hemidesmosomes between the basal region of the cells and the underlying collagen layer (Figure 8F).
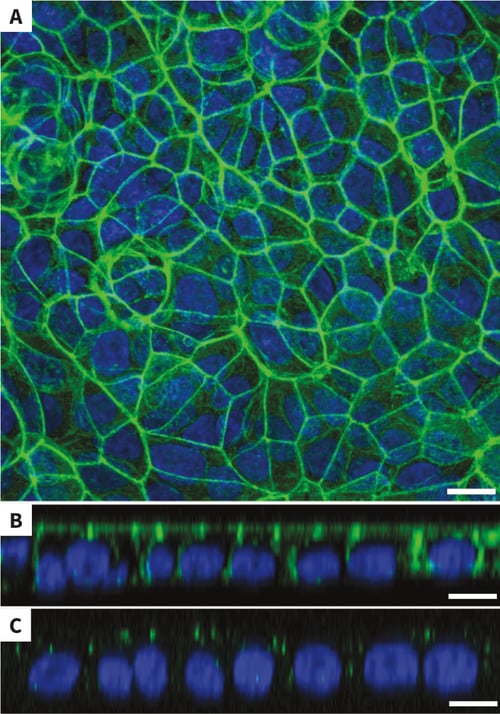
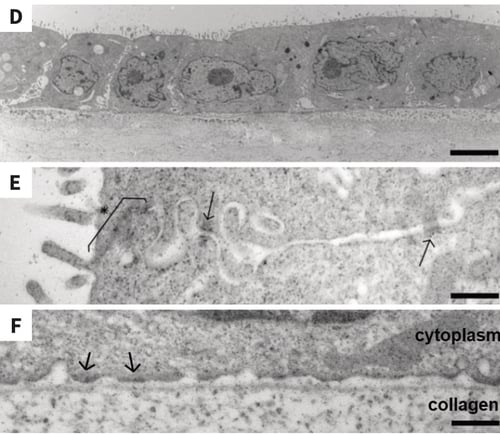
Figure 8: MDCK cells on collagen-coated Alvetex Scaffold form a confluent mono-layer with cell-cell junctions. Confocal immuno- fluorescent microscopy of phalloidin-stained wholemounts, showing that MDCK cells grown for 10 days in collagen-coated Alvetex Scaffold form a confluent mono-layer (A), with lateral phalloidin staining (B) and apical occluding staining (C). TEM imaging of the cells at the same time point clearly show evidence of regular cuboidal morphology (D), cell-cell junctions composed of both apical tight junctions (asterix and bracket) and desmosomes (arrows) (E), and basal hemidesmosomes (F). Alvetex Scaffold discs presented in 6-well insert in 6-well plate format. Cells were fixed in PFA, processed for phalloidin staining and viewed by confocal microscopy (A, B, C) or fixed in Karnovsky’s solution, embedded in LR white resin, sectioned (70 μm) and viewed by TEM (D, E, F). Scale bars: 10μm (A), 8 μm (B, C), 4 μm (D), 400 nm (E, F).
Taken together, these data again demonstrate that collagen-coated Alvetex Scaffold is highly suitable for the in vitro culture of epithelial monolayers that can then be used as such or as part of more complex co-culture models.
Conclusions and Applications
The combination of epithelial monolayer cell culture and collagen I coating in Alvetex Scaffold:
- Controls the promotion of either carcinoma or epithelial culture
- Promotes the formation of tight junctions and desmosomes
- Achieves differentiation of intestinal epithelial mono-layer
- Achieves formation of tubules in highly porous 3D environment
Alvetex Scaffold is a versatile 3D culture substrate which can be routinely used for the culture of epithelial monolayers, with or without prior layering with appropriate ECM. This option for surface coating, combined with Alvetex Scaffold’s highly porous structure and increased thickness, allows the development of co-culture set-ups without compromising on solute transport, which makes it ideal for furthering simple epithelia models into more complex mucosal models incorporating multiple cellular lines as well as ECM.
References
- Balimane PV et al. 2006. Current industrial practices of assessing permeability and P-glycoprotein inter-action. AAPS J. 8(1):E1-13.
- Bosman FT et al. 1993. Epithelial-stromal interactions in colon cancer. Int J Dev Biol. 37(1):203-11.
- Chantret I et al. 1988. Epithelial polarity, villin ex-pression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 48(7):1936-42.
- Cunha GR et al. 2004. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 67(5):417-34.
- Duell BL et al. 2011. Epithelial cell coculture models for studying infectious diseases: benefits and limitations. J Biomed Biotechnol. 2011:852419.
- Gaillard JL et al. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 55(11):2822-9.
- Horowitz JC and Thannickal VJ, 2006. Epithelial-mesenchymal interactions in pulmonary fibrosis. Semin Respir Crit Care Med. 27(6):600-12.
- Huber AR and Weiss SJ, 1989. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 83(4):1122-36.
- Koshida Y et al. 2006. Interaction between stromal fibroblasts and colorectal cancer cells in the expression of vascular endothelial growth factor. J Surg Res. 134(2):270-7.
- Leonard F, et al. 2010. A Three-Dimensional Coculture of Enterocytes, Monocytes and Dendritic Cells To Model Inflamed Intestinal Mucosa in vitro. Mol Pharmaceutics 7(6): 2103-19.
- Niu YN and Xia SJ, 2009. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl. 11(1):28-35.
- Parrinello S, et al. 2005. Stromal-epithelial interact-ions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 118(3):485-96.
- Raghavan S, et al. 2010. Decoupling diffusional from dimensional control of signaling in 3D culture reveals a role for myosin in tubulogenesis. J Cell Sci. 123(17):2877-83.
- Raymond M, et al. 2012. IL-1β stimulation of CCD- 18co myofibroblasts enhances repair of epithelial mono-layers through Wnt-5a. Am J Physiol Gastrointest Liver Physiol. 303(11):G1270-8.
- Spoettl T, et al. 2007. Role of soluble factors and three-dimensional culture in in vitro differentiation of intestinal macrophages. World J Gastroenterol. 13(7):1032-41.
- Svensson L, et al. 1991. Symmetric infection of rota-virus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 65(8):4190-7.
- Virag P, et al. 2012. Superior cytotoxicity and DNA crosslink induction by oxaliplatin versus cisplatin at lower cellular uptake in colorectal cancer cell lines. Anticancer Drugs. 23(10):1032-8.
- Visco V, et al. 2009. Human colon fibroblasts induce differentiation and proliferation of intestinal epithelial cells through the direct paracrine action of keratinocyte growth factor. J Cell Physiol. 220(1): 204-13.
- Wang AZ, et al. 1990. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 95(1):137-51.
- Willemsen LE, et al. 2002. A coculture model mimicking the intestinal mucosa reveals a regulatory role for myofibroblasts in immune-mediated barrier disruption. Dig Dis Sci. 47(10):2316-24.
- Zemans RL, et al. 2011. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci. 108(38):15990-5.
- Zuk A and Matlin KS, 1996. Apical beta-1-integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J Cell Sci. 109(7):1875-89.
Appendix: Materials and Methods
Detailed protocols for Wax and Resin Embedding, sectioning, H&E and Toluidine Blue Staining, Immunocytochemistry and Confocal Fluorescent Imaging are available on https://www.reprocell.com/resources/protocols.
Cell culture
Human colon carcinoma Caco-2 cells were obtained from ATCC (HTB-37) and were maintained in high-glucose (4.5 g/l) DMEM, supplemented with 20 % FCS, 0.1 mM non-essential amino acids, 10 mM HEPES buffer (pH = 7.4), 2 μM l-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. Human normal colon CCD-18Co fibroblasts were obtained from ATCC (CRL-1459) and were maintained in high-glucose (4.5 g/l) DMEM, supplemented with 10 % FCS, 2 μM l-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. Canine kidney MDCK cells were obtained from ECACC (84121903) and were maintained in MEM, supplemented with 10 % FCS, 0.1 mM non-essential amino acids, 2 μM l-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin.
Collagen I layer monoculture
Collagen I was layered on the top surface of Alvetex Scaffold 6-well inserts (product code AVP004-12S) presented in 6-well plates according to the dedicated ‘thin gel collagen coating’ protocol available on protocols . Briefly, Alvetex Scaffold inserts were prepared by ethanol wetting followed by two PBS rinses. The second PBS rinse was aspirated before 200 μL of 2 mg/ml rat tail collagen I solution (BD Biosciences, cat # 354236, see Alternate Gelation Procedure for BDTM Collagen I, Rat tail (PDF) for full instructions) were carefully added onto the surface of each insert, after which the inserts were returned to the 37°C, 5 % CO2 cell culture incubator for 3h to allow the gel to set. Each well was then flooded with 10ml of cell culture medium and returned to the cell culture incubator overnight.
The following morning, cells were prepared by trypsinising from the cell culture plastic and adding fresh medium. Cells were counted on a haemocytometer, centrifuged and resuspended in a sufficient volume of medium to give a concentration of either 0.5 million cells/ml (Caco-2) or 0.25 million cells/ml (MDCK). Medium was fully removed from each well, replaced with 5ml fresh medium outside the insert and cells were inoculated onto the surface of the collagen-coated discs as a 1 ml solution, i.e. either 0.5 million cells (Caco-2) or 0.25 million cells (MDCK) per insert without medium contact between the inside and outside of the insert. Seeded inserts were returned to the cell culture incubator to allow cell attachment overnight, after which the medium was removed and 10 ml fresh medium were added to each well, i.e. connected medium between the inside and outside of the insert. Cultures were maintained for up to 14 days (Caco-2) or 10 days (MDCK) with a full medium change every 2 days.
Collagen I layer co-culture
Alvetex Scaffold 6-well inserts presented in a 6-well plate were prepared for seeding with a 70% ethanol wash (10mL per well) and subsequent media washes (twice with 10ml). CCD-18Co fibroblasts were seeded at a density of 1 × 106 cells in 200 μL per insert. Enhanced CCD-18Co medium (high-glucose (4.5 g/l) DMEM supplemented with 10 % heat-inactivated FCS, 0.1 mM non-essential amino acids, 2 μM L-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin) was added to each well to a total volume of 7 ml taking care not to dislodge cells from Alvetex Scaffold, i.e. feeding separately from above and below. Plates were re-incubated and medium was added to a full complement of 10 ml per well the following morning. Cultures were maintained for 14 days, with weekly medium changes. After 14 days, medium was removed down to an air-liquid interface, i.e. feeding from below only, and 300 μL of 2 mg/ml rat tail collagen I solution (BD Biosciences, cat # 354236, see Alternate Gelation Procedure for BDTM Collagen I, Rat tail (PDF) for full instructions) were added to the surface of each insert. Plates were re-incubated for 3h to allow the collagen gel to set, after which fresh medium was added up to 10 ml per insert and plates were re-incubated overnight. The following day, medium was removed down to air-liquid-interface and Caco-2 cells which had been pre-accommodated to enhanced CCD-18Co medium for at least two passages on 2D were seeded at a density of 0.5 × 106 cells in 1 ml per insert over the surface of the collagen gel. Plates were re-incubated overnight to allow cells to attach, after which fresh medium was added up to 10 ml. Co-cultures were maintained for a further 5 days and the culture medium changed every 2 days.
Characterisation of enterocytic differentiation in Caco-2 cells
Cultures were processed for H&E staining, Immunocytochemistry staining, Toluidine Blue staining and Transmission Electron Microscopy (TEM).
For Immunocytochemistry, Caco-2 cultures grown on Alvetex Scaffold discs were processed as wholemounts, i.e. without any embedding or sectioning. After 10 days’ culture, Alvetex Scaffold discs were separated from their inserts, washed twice in PBS and fixed in PFA before processing as described for wholemounted discs in the ‘Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex Scaffold’ protocol available on https://www.reprocell.com/resources/protocols-alvetex. Filamentous actin was detected using Acti-stain™ 488 phalloidin (Cytoskeleton inc.), tight junctions were detected using an anti-occludin monoclonal antibody conjugated to AlexaFluor® 488 (clone OC-3F10, Life Technologies) and nuclei were counterstained with Hoechst 33342 (Molecular Probes), following manufacturers’ instructions specific to each antibody/stain. Stained wholemounts were then imaged on a Zeiss LSM510 meta confocal microscope.
For TEM, after 14 days’ culture, Caco-2 cultures grown on Alvetex Scaffold discs were separated from their inserts, washed twice in PBS and processed for resin embedding as described in the ‘Processing Alvetex Scaffold 3D Cultures for Resin Embedding And Toluidine Blue Staining for Light Microscopy’ protocol available on protocols. 70 nm sections were produced using a Reichert Ultracut S Ultramicrotome, sequentially stained with uranyl acetate and lead citrate and viewed with an Hitachi H7600 TEM.