Histology (5): Processing Alvetex® Scaffold 3D Cultures for Scanning Electron Microscopy (SEM)
● Download this protocol as a PDF (2.8 MB)
1. Introduction
Scanning electron microscopy (SEM) is becoming a popular method for visualisation of cultures grown in 3D. SEM is a form of electron microscopy where images are obtained by scanning samples using a high-energy beam of electrons. As the samples are scanned the electrons interact with atoms located on the sample surface, and it is these interactions that are detected, and processed, leading to high-resolution images depicting the samples’ topography and composition.
For conventional imaging, SEM samples must be electrically conductive and completely dry (due to the specimen chamber being at high vacuum).To achieve this, samples usually undergo chemical fixation to preserve and stabilise their structure. Standard SEM fixing solutions are Karnovsky’s fixative followed by a second fixing in osmium tetroxide, which increases the bulk conductivity of the sample. Samples are completely dried using a critical point dryer, and finally, fixed samples are sputter coated with gold to further enhance their conductivity.
Alvetex Scaffold can easily be processed like a standard tissue sample, allowing established methods to be followed with excellent results. An example protocol is outlined below. Imaging of the specimens will need to be optimised according to the specifications of the scanning electron microscope used.
2. Materials and Methods
2.1. Materials required
- Karnovsky’s fixative (2 % paraformaldehyde; 2.5 % glutaraldehyde in 0.1 M phosphate buffer pH 7.4. (for full recipe detail see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures.)
- Dehydration ethanols (30 %, 50 %, 75 %, 95 %, 100 %)
- 1 % buffered osmium tetroxide (1 % osmium tetroxide solution; 0.1 M phosphate buffer (pH 7.4). (For full recipe details see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures.)
- 0.1 M phosphate buffer pH 7.4
- Basic equipment: spade forceps to handle 3D cultures, scalpel (to trim Alvetex Scaffold cultures if required), disposable pipettes.
- Specialised equipment to prepare samples for SEM: critical point dryer, sputter coater.
2.2. Protocol
- Aspirate off the medium and carefully wash the 3D culture twice with PBS.
- Remove well inserts from cradle and carefully cut small samples (2-3 mm square) from the scaffold disc.
- Immerse samples in Karnovsky’s fixative at 4 °C for 90 minutes. The ratio of fixative volume to specimen size should be approximately 20:1.
- After fixation, aspirate the fixative into a waste container for disposal.
- Wash the specimen twice for 2 minutes in 3 mL 0.1 M phosphate to remove excess fixative, discarding waste liquid.
- Post fix samples in 1 % buffered osmium tetroxide for 90 minutes at 4 °C, then remove the osmium tetroxide solution.
- Add 20× the specimen volume of 30 % ethanol. Leave to equilibrate for 5 minutes preferably on a rotating wheel. Pour off the ethanol and discard. Repeat twice.
- Repeat step 2.7 with 50 %, 75 %, 95 % and then absolute ethanol. The sample can be stored in excess absolute ethanol.
- Follow the manufacturer’s instructions to completely dry the specimen using a critical point dryer.
- Follow the manufacturer’s instructions to gold plate the sample using a sputter coater. The gold coating will increase the sample’s conductivity and consequently improve image quality.
- The samples are now ready for imaging using a conventional scanning electron microscope.
3. Example Results
Figure 1 below shows an example of a 3D Alvetex Scaffold culture imaged using the SEM technique.
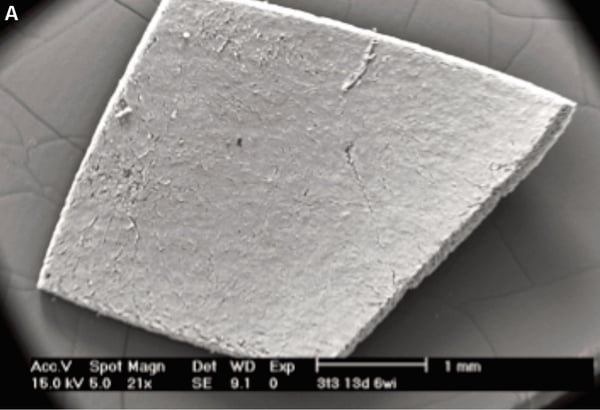
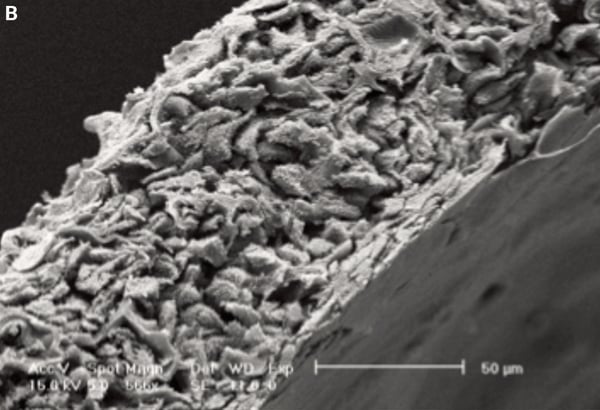
Figure 1. Detailed structure of 3D cell cultures can be visualised using scanning electron microscopy. Inspection of pieces of Alvetex Scaffold at low magnification shows homogeneous coverage by cultured cells (A). Higher magnification imaging in this transverse section reveals cells growing throughout the scaffold (B). Scale bars 1 mm (A) and 50 µm (B).