Collagen I Thin Gel Coating of Alvetex® Scaffold
1. Introduction
Extra-cellular matrix (ECM) coating of in vitro culture surfaces is commonly used to enhance cell-substrate adhesion, encourage cell-matrix signalling and to protect shear-sensitive cells in perfusion devices. The following protocol outlines how to coat the top surface of Alvetex Scaffold membranes with a thin gel layer of collagen I. This method can be used to promote adhesion and culture of a monolayer of epithelial cells at the surface of Alvetex Scaffold, either as a mono- culture for diffusion assays, or as co-culture for tissue engineering (e.g. a surface epithelial compartment layered on top of a fibroblastic compartment grown inside Alvetex Scaffold). The data shown exemplify the application of this protocol to grow colorectal adenocarcinoma CaCo-2 cells on collagen I coated Alvetex Scaffold for 4 days in 6 well inserts (AVP004).
2. Method
- Prepare Alvetex Scaffold for coating by first treating with 70 % ethanol followed by two PBS washes as described in the Alvetex Scaffold Quick Start protocol. Leave Alvetex Scaffold in the second PBS wash until ready to apply the collagen solution.
- Dilute rat tail collagen I (Corning, 354236) to a concentration of 2 mg/mL using cell culture grade water and adjust the pH with NaOH according to manufacturer’s instructions.
Note: Ensure all reagents are maintained on ice and use pre-chilled pipette tips to perform the dilution and subsequent application onto Alvetex Scaffold.
-
Aspirate the second PBS wash from Alvetex Scaffold disc. Do not allow the scaffold to dry out. Immediately and carefully pipette an appropriate volume of the diluted collagen solution onto each disc. Depending on the desired thickness of the collagen layer, use 100-400 μL of collagen solution per disc for the 12 well plate format (AVP002) and the 6 well insert format (AVP004) or 75-200 μL of collagen solution for the 24 well plate (AVP006) and 12 well insert format (AVP005).
-
Replace plate lids and leave to stand for 3 hours at 37 °C.
-
Remove excess fluid from Alvetex Scaffold in well insert format by gently tapping the plate or Petri dish on the worktop. Check that no residual fluid is hanging from the base of the well inserts. Aspirate to remove any residual gelling agent from the bottom of the wells. If using Alvetex Scaffold in 24 or 12 well plate format, tilt the plate and gently aspirate any excess fluid from the edge of the wells.
-
Keep collagen-layered Alvetex Scaffold hydrated by flooding the membrane with medium until cells are ready for seeding.
-
Prepare cells for seeding in the appropriate culture media and seed directly on the wet collagen-layered Alvetex Scaffold membrane, in the volumes relevant for the Alvetex Scaffold product format used. See the Alvetex Scaffold Quick Start protocol for details.
3. Example data: collagen I thin gel coating of Alvetex Scaffold
Pre-coating of Alvetex Scaffold discs with a 2 mg/mL collagen I solution resulted in a layer of even thickness (Figure 1) and covering the whole surface of the scaffold (Figure 2). The integrity of the collagen I layer was demonstrated by the absence of Crystal Violet dye leakage when the collagen-coated Alvetex Scaffold discs were placed in a Side-Bi-Side PermeGear diffusion chamber (Figure 3).
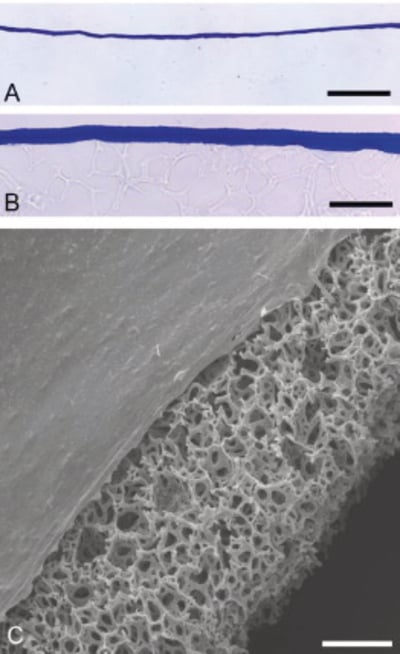
Figure 1. Thickness of Collagen I Coated Alvetex Scaffold. A, B: Brightfield micrographs showing low (A) and high (B) magnification images of PhastGel® Blue R staining of sectioned 6 well insert (AVP004) coated with 100 μL of 2 mg/mL collagen I. C: Scanning electron microscopy showing sideview of collagen I 6 well insert (AVP004) coated with 100 μL of 2 mg/mL collagen I. Note the constant thickness and smooth surface of the collagen coating across Alvetex Scaffold. Scale bars: (A) 50 μm; (B) 8 μm; (C) 100 μm.
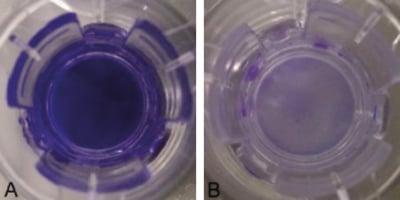
Figure 2. Uniform Coverage of Collagen I Coated Alvetex Scaffold. Brightfield micrographs showing PhastGel Blue R staining of whole 12 well inserts (AVP005) coated with 100 μL of 2 mg/mL collagen I (A) compared to uncoated controls (B). Note the complete coverage of the collagen-coated Alvetex Scaffold disc.

Figure 3. Integrity of Collagen I Coated Alvetex Scaffold. Crystal Violet Dye diffusion test in Side-Bi- Side PermeGear chamber using whole 12 well inserts (AVP005) either uncoated (A) or coated with 200 μL of 2 mg/mL collagen I (B) to separate donor (left) from recipient (right) chambers. Diffusion was assessed with mechanical agitation of both chambers. Timepoint: (A) 30 seconds after dye introduction, (B) 2 minutes after dye introduction. Note that the dye is excluded from the recipient chamber in the presence of collagen-coated Alvetex Scaffold, whereas it has freely entered the recipient chamber in the absence of collagen I coating.
4. Example Data: Growth of CaCo-2 Colorectal Adenocarcinoma Cell Line on Collagen I Thin Gel on Alvetex Scaffold
4.1. Cell Culture details
CaCo-2 cells (ATCC, HTB-37TM) were routinely maintained in T-75 flasks. CaCo-2 complete medium consisted of: high-glucose DMEM media supplemented with 20 % v/v FBS, 0.1 mM non-essential amino acids, 10 mM/L HEPES (pH 7.4), 2 mM L-glutamine and 100 U/mL Penicillin/ Streptomycin. Alvetex Scaffold 6 well inserts (AVP004) in 6 well plates, were layered with 200 μL of 2 mg/mL collagen I as described above. 5 mL medium was added to the bottom of each well, i.e. on the outside of the Alvetex Scaffold insert, taking care not to trap air bubbles underneath the insert. Cells were seeded on top of each disc at a density of 0.5 × 106 cells in 500 μL medium suspension and were left to attach overnight in an incubator (5 % CO2, 37 °C). The following day, medium was carefully added to each sample up to 10 mL per well. Culture medium was changed every two days. After 4 days and 10 days culture, samples were fixed, embedded, sectioned and stained for hematoxylin & eosin or toluidine blue, according to established protocols (see Alvetex protocols).
4.2. Results
Culture of CaCo-2 cells on collagen I coated Alvetex Scaffold resulted in an even cell monolayer of epithelial morphology. Apical structures characteristic of differentiated intestinal epithelium (microvilli), were evident after 10 days’ culture (Figure 4).
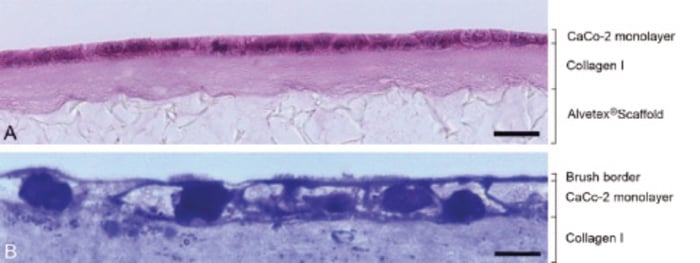
Figure 4. Epithelial Morphology of Caco-2 Cells Grown on Collagen I Coated Alvetex Scaffold.
A: Brightfield micrographs of H&E-stained paraffin-embedded sections showing CaCo-2 cells cultured for 4 days. Note that cells form a densely-packed monolayer. B: Brightfield micrographs of Toluidine Blue stained resin-embedded sections showing CaCo-2 cells cultured for 10 days. Note the presence of microvilli on the apical surface forming the brush border. Scale bars: (A) 25μm; (B) 8μm.