3D Bioengineered Model for Inflammatory Bowel Disease
Drug Discovery Assay – reference number: B125
Overview
| Tissue: | Bioengineered human IBD tissue |
| Target: | Study dependent |
| Control compound: | BIRB796, 5-ASA, prednisolone, tofacitinib |
| Study type: | 3D cell culture |
| Functional endpoint: | Inflammation, fibrosis/ remodelling, cytokine release, gene expression |
Effect of 10μm BIRB796 on IL-6 release. PCR study: n = 3-4 independent experiments (3 models of same condition pooled per experiment)
Testing Information
Assay Description
This experiment assesses whether test articles cause a reduction in inflammatory mediator release with BIRB796/5-ASA/Prednisolone/Tofacitinib as a reference compound. The specific results that will be provided are the effects of test articles on the levels of cytokine release in the culture medium.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on the diluent vehicle used. Stock solutions are prepared in distilled water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
A minimum of triplicate per condition with a maximum of 72 models per experiment. Several experiments can be run in a single study to increase model throughput.
Study Outline
Rationale and Experimental Design
To form tissue layers that mimic inflamed IBD mucosa, our researchers culture three different cell types in Alvetex® Scaffold inserts. Fibroblasts are seeded first to generate abundant endogenous ECM and growth factors to support other cell types. Differentiated immune cells are then added to create a viable immune component and form a relevant lamina propria compartment. As these immune cells contain an NFκB reporter, their activation can be easily quantified using a colorimetric assay. Finally, epithelial cells are added to create a confluent microvilli brush-border, mimicking the lining of the human intestine.
Once the cells have matured, the model can be challenged with inflammatory stimuli to produce an inflammatory response. Lipopolysaccharide (LPS) is regularly used as a standard stimulus but we can also use cytokines to induce an inflammatory response. If testing the prophylactic effect of compounds the model is stimulated following drug additions. If testing the acute effects of test articles the model will be stimulated from the beginning of the culture period. Each model can be cultured for up to 96 hours after establishment.
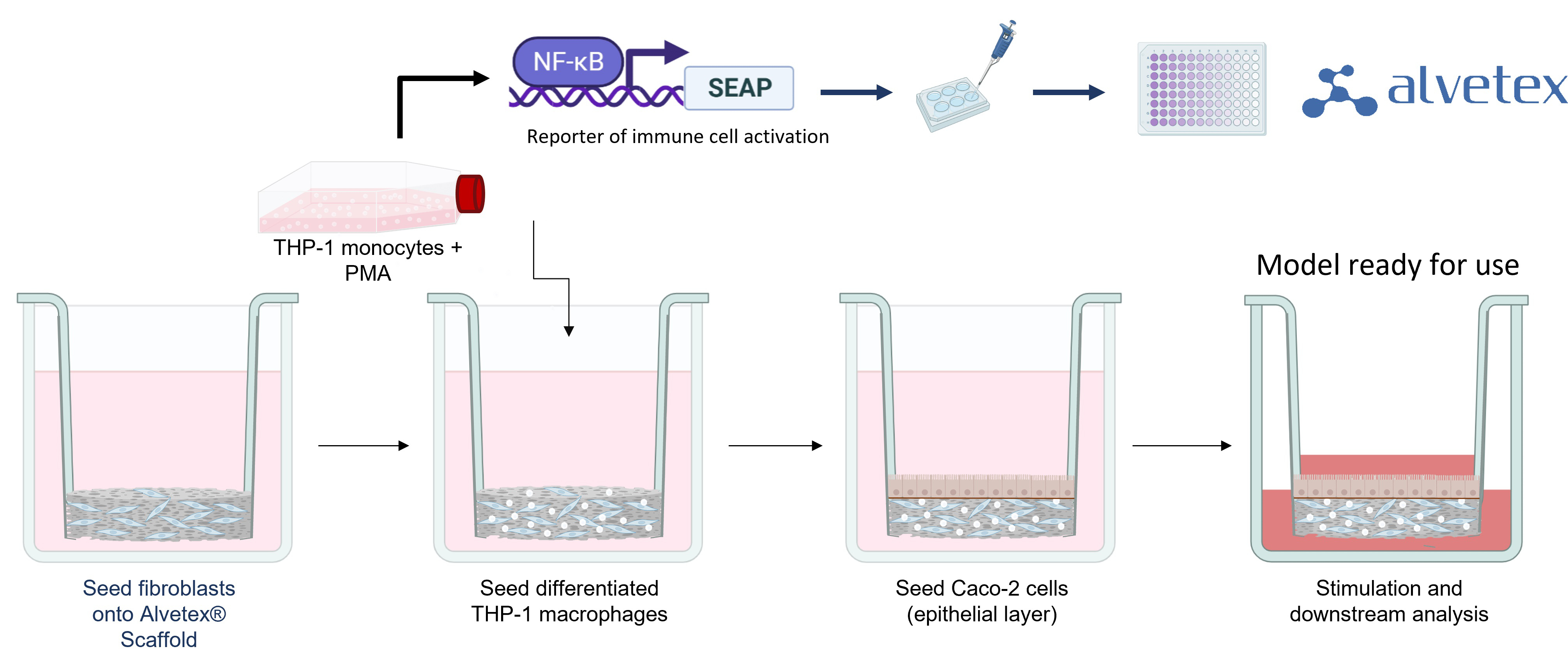
Addition of Test Articles
Test articles can be added directly to the culture medium (no carrier required). It is recommended a minimum of triplicates should be used for each condition. An example of the conditions assessed for three test agents would be as follows:
- Non-stimulated control
- Stimulated control
- Test agent 1
- Test agent 2
- Test agent 3
- Positive control
Endpoint Analysis
There are a range of endpoints that can be used with this model including inflammation, fibrosis, and barrier disruption:- Immune response: Quantification of NFkb activation, cytokine and chemokine production, inflammatory marker production, and pro-inflammatory status.
- Barrier integrity: Histological assessment, ICC/IHC staining, junction analysis, junction quantification by PCR and WB, and EM analysis
- Lamina-propria compartment: ECM quantification, ICC/IHC staining, MMP/TIMP production, cytokine/chemokine production, and marker quantification
We also offer transport studies using this model; if you are interested in measuring changes in permeability and transepithelial electrical resistance (TEER) You can find out more about these studies here.
Example Data
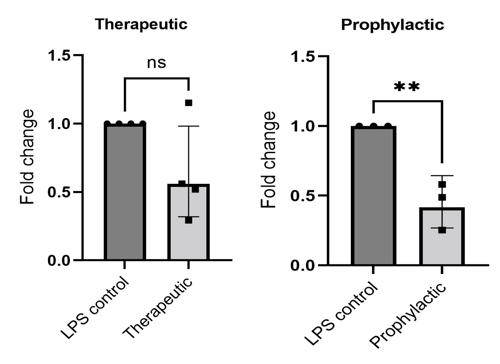
Inflammation: 72h Birb796 10 µM treatment reduces cytokine production
| A | 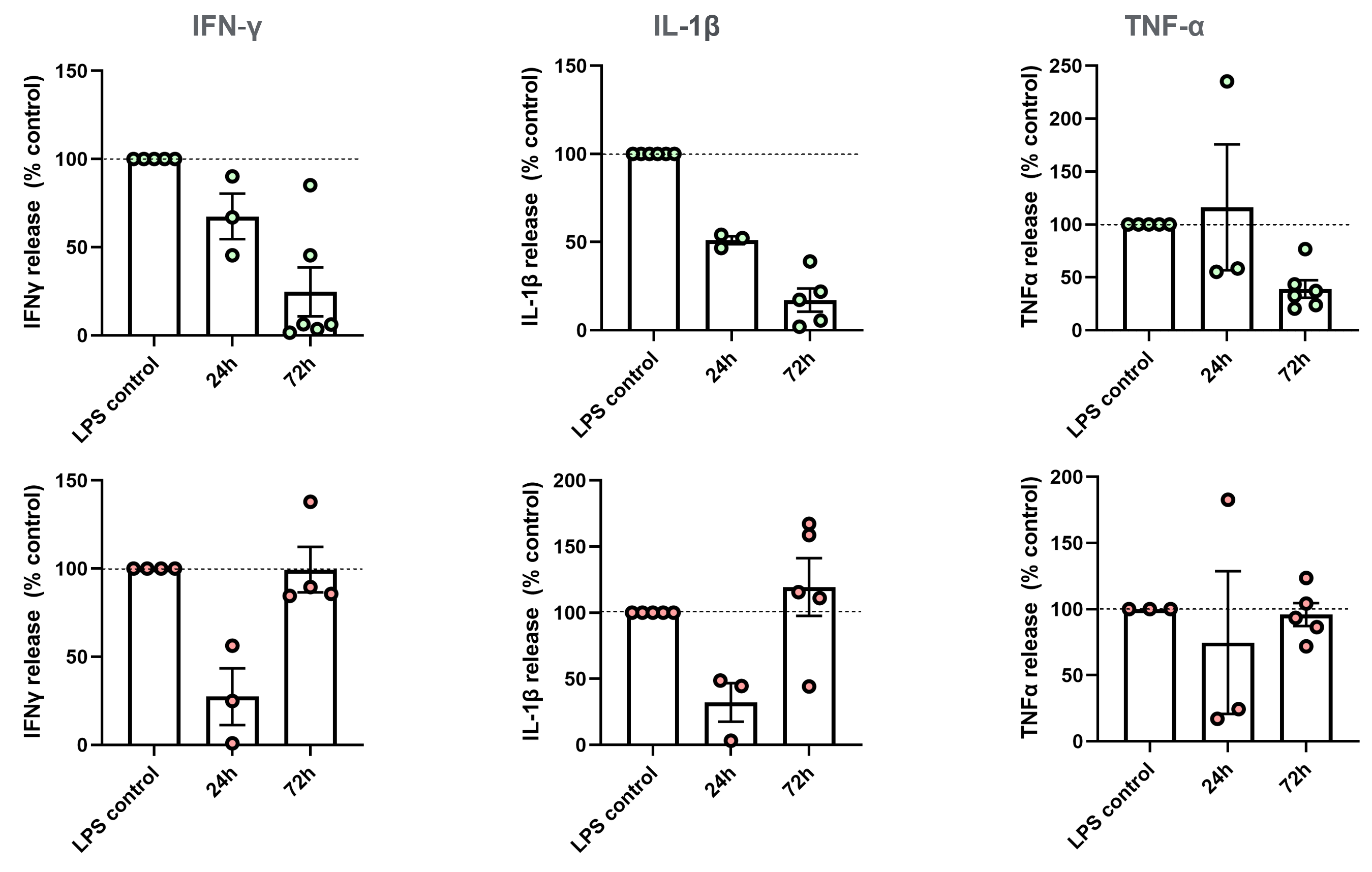 |
| B |
72h Birb796 10 µM treatment using either (A) prophylactic or (B) therapeutic approaches, reduces the release of proinflammatory cytokines commonly upregulated in IBD. Values presented as mean±SEM. 1 technical replicate performed per sample, 1 sample for each condition analyzed per experiment. n=3-5 independent experiments (3 for 24 hours and 5 for 72 hours).
Inflammation: 72h Birb796 10 µM treatment reduces chemokine production
| A | 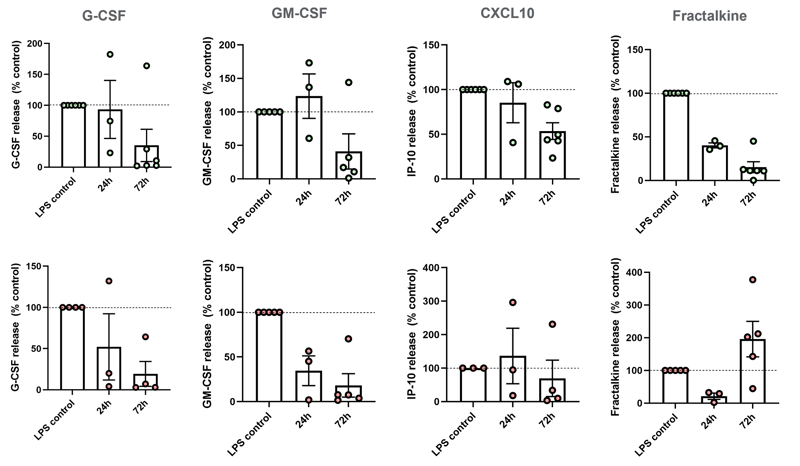 |
| B |
72h Birb796 10 µM treatment using either (A) prophylactic or (B) therapeutic approaches, reduces release of proinflammatory chemokines commonly upregulated in CD or UC. BIRB796 reduces IBD-related chemokine production using prophylactic and therapeutic approach. n=3-5 independent experiments (3 for 24 hours and 5 for 72 hours)
Inflammation: 72h BIRB 796 10 µM treatment reduces (A) CXCL8 and (B) IL6 gene expression.
| A | 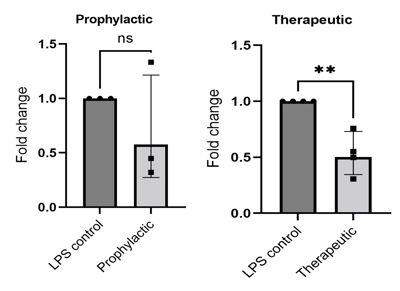 |
| B | 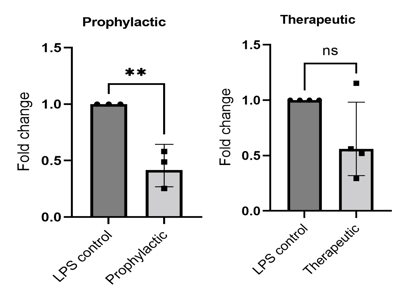 |
72h BIRB 796 10 µM treatment reduces (A) CXCL8 and (B) IL6 gene expression. Following treatment with drug compounds, we have assessed inflammatory biomarkers associated with IBD through cytokine quantification as well as qPCR quantification. Two examples of qPCR data are shown here. CXCL8 and IL6 genes are significantly reduced by 10 µM BIRB796 treatment. PCR N=3-4 independent experiments. Dot represents each experimental repeat
Barrier Disruption: BIRB 796 protects against epithelial disruption
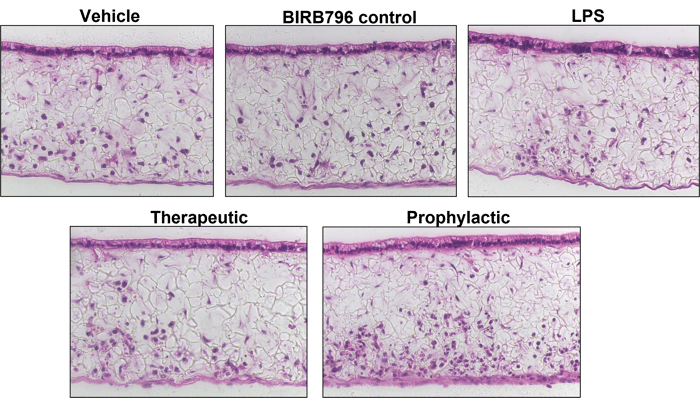
Histology suggests 72h BIRB 796 protects against epithelial disruption. Models treated with BIRB796 for 72 hours show fewer signs of inflammatory disruption: fewer epithelial breaks, more polarized epithelium. Immune cells appear more abundant in treated models, suggesting immune cell migration is reduced as a result of BIRB 796 treatment
Histological assessment performed after drug treatments.
Barrier disruption: BIRB 796 reduces phosphorylation of p38-MAPK
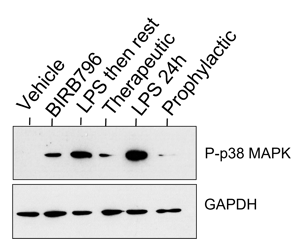
Western blot (WB) confirmation of histology that BIRB 796 reduces phosphorylation of p38-MAPK and is having the desired effect in models.
ECM Remodelling: 72h BIRB 796 10 µM treatment reduces MMP release within stimulated models
| A | 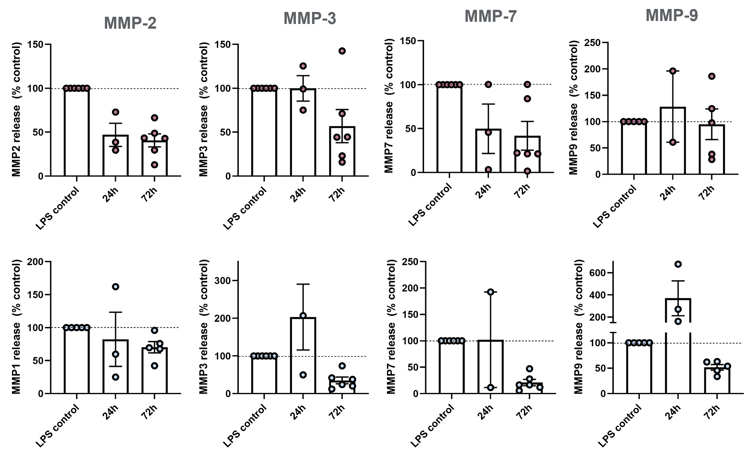 |
| B |
IBD models can be used to assess efficacy of drugs in reducing ECM-remodelling enzymes involved in IBD pathophysiology (n=3-5). MMP-2, 3, 7, and 9 are upregulated in IBD patients, with MMP-9 being the most abundant MMP in IBD tissue and serum.


