When you are planning a project involving stem cell reprogramming, there are several factors to consider when choosing a reprogramming technology and a service provider. This post will highlight 5 questions you may want to answer before choosing a service provider or reprogramming technology.
There are many available technologies for generating iPSCs, each with their strengths and weaknesses. It is important to make sure that the core technology matches with the goals of your project. Important variables to consider include time to usable iPSCs, quality of the iPSCs, and clinical relevance of the technology.
Why use RNA reprogramming?
Using mRNA for reprogramming provides the optimal combination of efficiency, speed, quality, and clinical relevance.
RNA reprogramming:
-
Provides up to 4% efficiency - the highest number of iPSC colonies per starting target cell of any common reprogramming method. This can mean the difference between success and failure of the project when reprogramming target cells from actual patients.
-
Generates high quality iPSCs in the shortest time, allowing the project to quickly proceed to the next step. Stem cells reprogrammed with mRNA do not retain reprogramming vectors, meaning that no time-consuming vector screening is required, as mRNA is cleared from the cell within 18 to 24 h after transfection.
-
Carries the lowest rates of genetic abnormalities such as aneuploidy, compared to the starting target cell, at the chromosomal level.
-
Displays low batch-batch variation, ensuring that work won’t need to be repeated as you move from research to clinical development. Therapeutic mRNA is currently being investigated in clinical trials in several disease areas[1], showing the synthesis of clinical grade RNA is readily accessible.
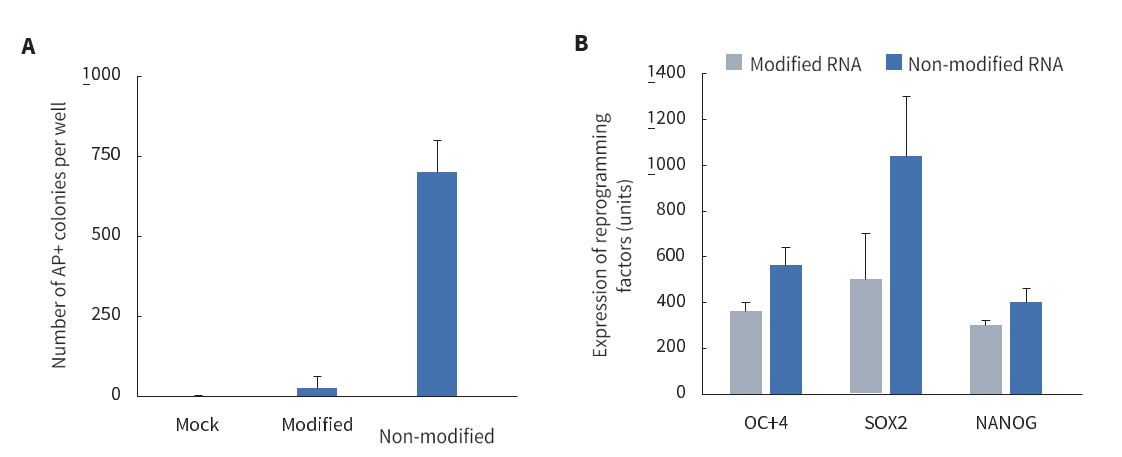
Figure 1. Non-modified bases are used in the mRNA vector, providing higher translation efficiency (A) and increased reprogramming efficiency (B).
StemRNA 3rd Gen Reprogramming Technology
Stemgent® StemRNA™ 3rd Gen Reprogramming Technology uses only RNA to reprogram somatic cells into iPSCs. The RNA reprogramming cocktail contains six reprogramming mRNAs (OCT4, SOX2, KLF4, c-MYC, NANOG, LIN28), three immune ablating mRNAs (E3, K3 and B18), and a proprietary mixture of microRNA.
-
Using 6 reprogramming factors in the cocktail ensures the highest efficiency and allows the technology to reprogram cells from a variety of starting sources, including skin (fibroblasts), blood and urine. The cocktail employs mRNAs with natural, non-modified nucleosides, rather than the modified ones typically used for synthetic mRNA, to ensure high translation and reprogramming efficiencies.
Read: Tissue Sourcing for the Development of iPSC-derived Disease Models →
- Use of the immune ablating mRNAs reduces the toxicity associated with cellular exposure to RNA, allowing for sustained translation of the reprogramming factors (Figure 1). This results in reduced cellular toxicity, increased translation efficiency, reduced degradation of the reprogramming mRNAs, and no need for supplementation with B18-R protein compared to common mRNA approaches.
-
Use of a microRNA cocktail primes cells for reprogramming and increases efficiency for the difficult to reprogram patient samples which are frequently encountered in real-world reprogramming projects.
The unique combination of these three components into our platform provides the highest efficiency and most robust reprogramming technology available.
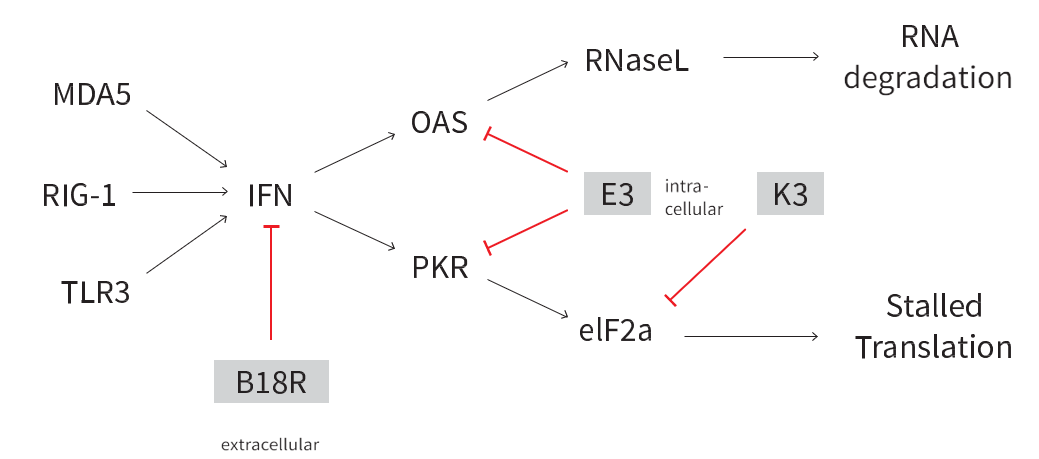
Figure 2. The natural cellular defense mechanisms against foreign RNAs is blocked using the StemRNA 3rd Gen technology, by inhibiting Interferon α, RNA degradation, and stalled translation.
Is commercial sale of iPSC products allowed and what is my Freedom to Operate?
If the final goal of your iPSC program is commercialization, make sure that your service provider will work with your needs and timelines.
With REPROCELL’s services, internal R&D use or commercial sales of products or services using differentiated iPSCs or derivatives is allowed. There are no upfront fees, annual maintenance fees or royalties if the customer does not commercialize the product. Distribution (passing and sale) of undifferentiated iPSCs, or clinical use of iPSCs or derivatives requires additional agreements directly through REPROCELL. Please contact us for more details.
What are the details of the service package?
Not all service project quotes are the same. When comparing different service quotes, make sure that quotes provide a comparable level of service. Points to consider include
-
Where will the target cells (patient cells) come from?
-
What level of purification will I get with the iPSCs (individual clones or a multiclonal pool)?
-
What kind of QC data will I get with the cells?
-
Is there flexibility to choose other (non-standard) things in the reprogramming project.
-
Will I be kept up to date on the progress of the project and any roadblocks encountered?
What does REPROCELL’s stem cell reprogramming service include?
At REPROCELL, every service project is milestone-based and customizable to meet your needs. For iPSC services, we can perform donor tissue collection, sample quarantine, derivation of primary cell lines, RNA-based iPSC reprogramming, expansion, characterization, cryopreservation, and differentiation into particular cell types. Our standard quality control package includes mycoplasma, infectious disease check, STR analysis and live staining for certain pluripotency markers.
Additional characterization services can be modified to fit the specific needs of the project. Examples of assays include karyotyping, cell banking, genome-editing, teratoma formation, CGH/SNP arrays, and more. REPROCELL also assigns a dedicated study director to be your single point of contact handle logistics and keep you updated about the status of your project.
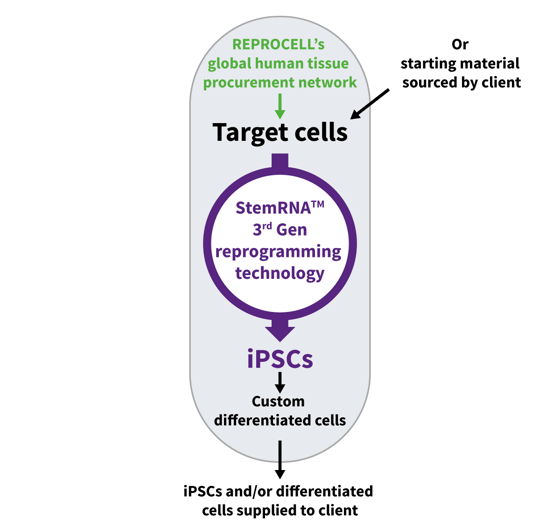
Figure 3. REPROCELL provide a comprehensive reprogramming service
About REPROCELL’s Custom iPSC Service Group
REPROCELL’s Custom iPSC Reprogramming Service group uses our Stemgent® StemRNA™ 3rd Gen technology to generate integration-free, RNA-based, clinically relevant induced pluripotent stem cells. We can start from your cells, or we can more-than likely source the cells you need through our global human tissue procurement network.
If your company or institution needs customized iPSCs for research purposes or for use in commercial products or services, please check us out – our Frequently Asked Questions document (free PDF download) may give you the answers you are looking for right now. Of course, you’re also welcome to contact us if there’s anything else you need to know.
References
- Heesen L et al. A first-in-human phase I/II clinical trial assessing novel mRNA-lipoplex nanoparticles encoding shared tumor antigens for potent melanoma immunotherapy. Annals Oncology 28:1 (2017)
Editors note: This post was originally published in 2018, and has since been updated for accuracy and comprehensiveness.





.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



