In translational medicine, research using primary patient samples is advantageous over cell lines as the expression of the protein under investigation is regulated by native elements in primary samples.
However, there are several concerns associated with using primary cells such as the limited proliferation of terminally differentiated primary cells. Furthermore, even under optimal culture conditions primary samples do not exhibit a stable phenotype in long term culture.
Animal models in drug discovery
To date, pre-clinical phases of drug development and the exploration of disease mechanisms has been based mainly on animal models. Besides the ethical concerns and financial issues involved in using animals in medical research, these models do not always demonstrate the same phenotype as those seen in humans because they do not replicate human pathophysiology. This can lead to false negative results when testing the toxicity of a compound [1].
Furthermore, when it comes to cancer studies some cancer-associated genes do not have a clear animal counterpart or have mutations that are too complicated to be engineered into the animal genome [2]. Also, compounds that demonstrate significant benefit in animal models may fail to show effectiveness in clinical trials in humans [3].
Considering all of these issues, the conventional drug discovery pathway is a very inefficient process with the majority of candidate drugs not reaching the market due to safety and/or efficacy issues.
Use of induced pluripotent stem cells (iPSCs) allows high throughput screening of therapies by providing disease-specific treatment using patient-specific models. This may reduce/refine the use of animal models and make the drug discovery pathway more efficient (Figure 1).
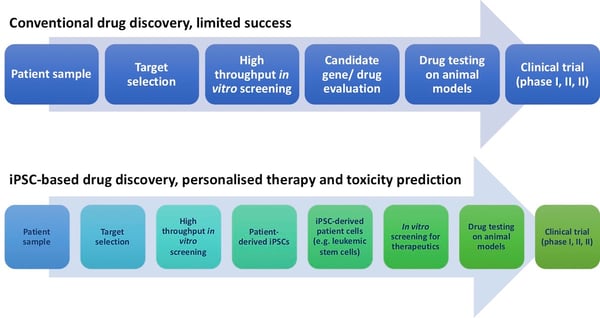
Figure 1. Conventional and iPSC-based drug discovery pathways
iPSCs in translational medicine and drug discovery
Following the initial work by Thomson and colleagues in 1998, embryonic stem cells (ESCs) were predicted to provide a powerful platform to interrogate various disorders as well as a limitless supply of cells for therapy and transplantation [4].
Ethical concerns surrounding the use of ESCs and the recent advances in using iPSC technology have made the human iPSC model a good approach [5].
The potential of iPSCs to give rise to all three germ layers is a cornerstone of their potential in regenerative medicine. This property of iPSCs has made them good models for ectodermal (neuronal disorders), mesodermal (cardiac disease) and endodermal (inherited metabolic disorders) lineage disorders (Figure 2).
iPSCs have also been used as models in studying cancer. Primary tumours obtained from patients and their derivative cell lines can help us understand late stage markers and cellular phenotypes; however they lack the possibility of studying early stages of the cancer in question, whereas, patient-derived iPSCs have the potential to represent the earliest stage of disease [6].
iPSCs have the potential to provide patient-specific scalable biologic material of various tissue types, useful in investigating the pathophysiology of rare disorders. These cells provide an unlimited source for the study of effectiveness and toxicity of pharmaceuticals by generating (healthy or diseased) tissue of interest. The renewability of these cells allows high throughput screening, making them desirable platforms for therapeutic drug screening and toxicological studies.
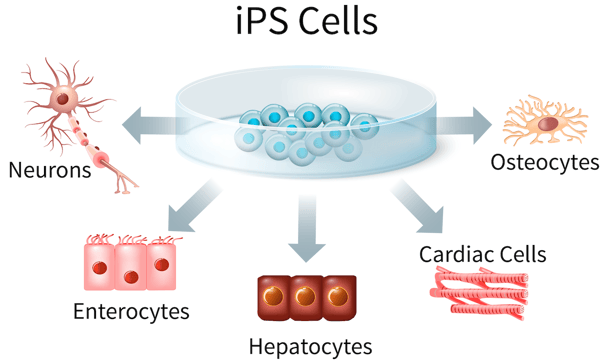
Figure 2. iPSCs are pluripotent which gives them the capacity of differentiating into three germ layers; endoderm, mesoderm and ectoderm. This makes them a suitable starting material to produce various patient-derived terminally differentiated cells
How REPROCELL can help you generate an iPSC model
Here at REPROCELL we provide services generating iPSCs from various tissue sources using our mRNA reprogramming technology. These cells can be differentiated into your desired tissue-specific cells for further studies. iPSCs may be the right model system for your project, you simply need to contact our experts for more information REPROCELL Tissue Network, Reprogramming and Molecular Services.
References
-
Gunaseel et al. Induced pluripotent stem cells as a model for accelerated patient- and disease-specific drug discovery. Curr Med Chem 17 (2010)
-
Kim et al. Upregulated hoxC4 induces CD14 expression during the differentiation of acute promyelocytic leukemia cells. Leuk Lymphoma 46 (2005)
-
Wichterle et al. What can pluripotent stem cells teach us about neurodegenerative diseases? Nat Neurosci 13 (2010)
-
Thomson et al. Neural differentiation of rhesus embryonic stem cells. APMIS 106 (1998)
-
Takahashi et al. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (2006)
-
Kim. Applications of iPSCs in Cancer Research. Biomark Insights 10 (2015)




.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



