Stemchymal®[1] is an allogenic stem cell therapeutic derived from the adipose tissue of healthy donors. Mesenchymal stem cells (MSCs) are isolated from the adipose tissue using a propriety cell processing system developed by Steminent Biotherapeutics[2]. The purified MSCs are then administered to patients via Intravenous (IV) infusion.
Stemchymal cell therapy for SCA3
Currently, Stemchymal® is being trailed for the treatment of Spinocerebellar Ataxia 3 (SCA3) a condition characterized by progressive cerebellar ataxia. So far, the treatment has been deemed safe for IV administration. You can discover the main findings from the phase I and phase II trials in our infographic below.
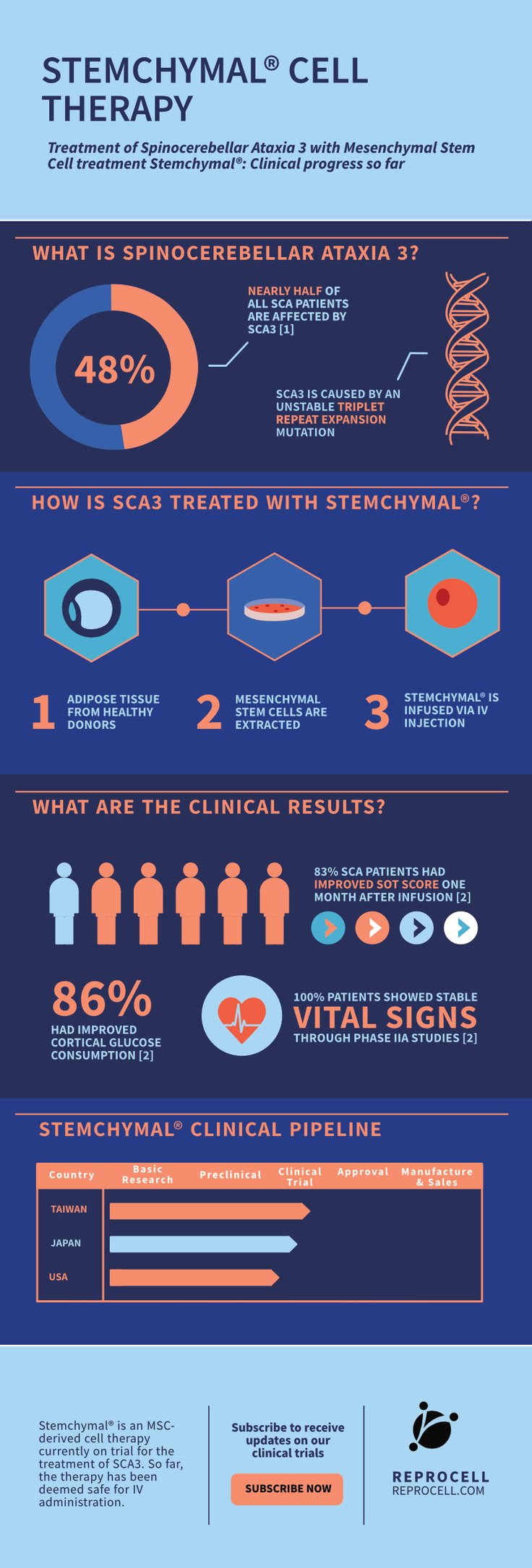
Stemchymal phase I/II clinical trials
In this infographic we have summarized the main findings from the Phase I/II clinical trials of Stemchymal®. We have also provided information on the disease this therapeutic aims to treat, and a timeline of how the drug is progressing clinically in different countries. Inquire now to find out more about our clinical capabilities →
References
- What is Stemchymal® - Steminent Biotherapeutics
- About Steminent - Steminent Biotherapeutics
- Tang et al. Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds. Arch Neuro 57:4 (2000)
- Tsai et al. Treatment of Spinocerebellar Ataxia 3 with Mesenchymal Stem Cell treatment Stemchymal. Cell Trans 26 (2017)




.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



