Today, clinical trials are conducted to aid our understanding of, and to assess new treatments for, medical conditions or disorders that affect the human population. The plethora of investigative research initiated following the emergence of the coronavirus, SARS-CoV-2, in 2019 and the resultant development, and worldwide roll out, of a life-saving vaccine is one example of our current global clinical trial landscape.
Considered the gold standard for medical research, the modern clinical trial is a well-controlled, but complex process that functions within a strict regulatory environment. Whilst it can be frustrating and time-consuming to navigate this research ecosystem, recognizing how clinical trial systems and processes have developed and evolved can provide some useful context for today’s scientists and clinicians.
In The Beginning
A clinical trial as we understand it today and as defined by the World Health Organisation “is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes.” This simple, succinct statement belies many years of intellectual and philosophical discussion and investigation that is peppered with numerous scientific discoveries, milestones, and advancements. Documented attempts to compare and observe patient health outcomes go back thousands of years with crucial landmarks in the history of clinical research defining the introduction of modern-day clinical trial tools such as: trial control; randomization; blinding; placebo; and, more recently, universal ethical standards and research regulation and oversight.1,2 This rich history and the lessons learned define the high-quality informative and varied research approaches that we see in clinical research today.
The United Kingdom Medical Research Council’s 20th century clinical trial of streptomycin for the treatment of pulmonary tuberculosis is often considered the birth of the modern-day clinical trial approach. The first truly randomized controlled trial in humans, it was ground-breaking in its design and conduct. However, the first documented study that loosely resembled the definition of a clinical trial was written and recorded in the Old Testament sometime between the 15th and 4th centuries BC. The first chapter of the Book of Daniel describes a comparative investigation of diet and health in a population. The Book accounts the King of Babylon – King Nebuchadnezzar’s mandate to his men to consume a diet of meat and wine in a bid to maintain perpetual health. Daniel and his brothers, all vegetarians, dissented to follow the King’s directive. In a bid to prove his point the King allowed them to consume a diet of legumes and water for several weeks and then he compared the overall general health of the two groups. His assessment was that Daniel, and his brothers, were healthier and so he altered the diet of his men accordingly. Although there may be historical inconsistencies and a lack of robust scientific confirmation, this was the first documented comparative clinical study and perhaps one of the first documented cases of a public health initiative.
Comparative and observational studies continued throughout the centuries – through the Greek and Roman eras, the Middle Ages, the Renaissance – to the 20th century. Over this historical timeframe, several seminal documented studies were to prove critical in driving the evolution of clinical research and advancements in clinical trial design, operation, and interpretation.
A Happy Accident: Trial of a Novel Therapy
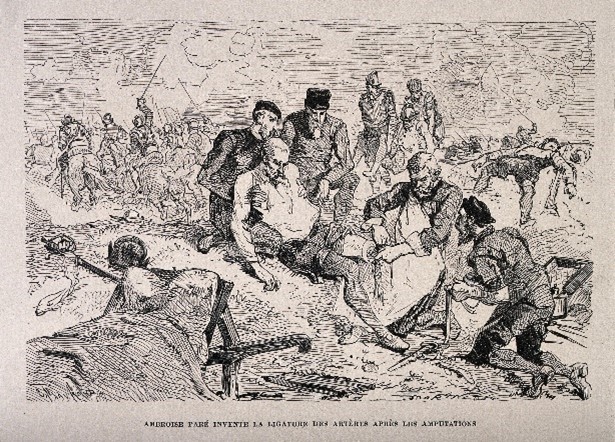
In 1537, whilst serving with the Mareschal de Motegni, the French military surgeon Ambroise Paré, inadvertently performed the first clinical trial of a novel therapy.3 Paré was responsible for the treatment of the battlefield wounded following the capture of the castle of Villiaine: the treatment convention of the time being application of a boiling elderberry oil and cauterization. When elderberry oil supplies ran out because of the vast number of wounded requiring treatment, he was forced to apply an alternative ‘ointment’ consisting of turpentine, egg yolk, and rose oil. Despite his reservations that soldiers would die due to the lack of heat and cauterization, he found that the ointment resulted in less pain, less wound irritation, and a general overall improvement in outcomes when compared to those treated with the boiling oil. Although unplanned, the ointment proved more effective than the standard of care treatment and thereafter, Paré avoided the use of cauterization. Paré’s accidental observation was the first real advancement of care through human experimentation and comparison and set the approach for many years to come amongst physicians and scientists.
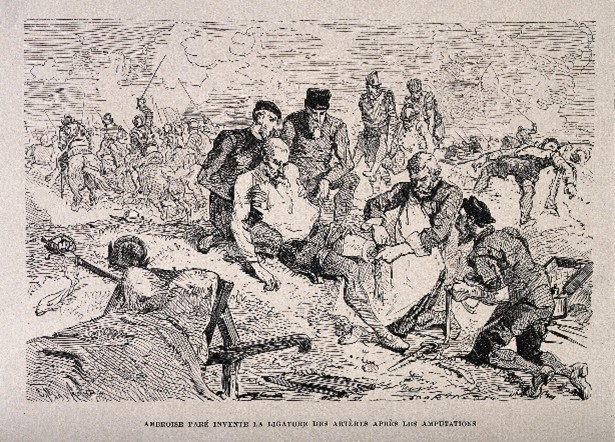 Figure 1: Ambroise Paré, on the battlefield using a ligature for the artery of an amputated leg of a soldier. Wood engraving by Charles Maurand after E. Morin. Credit: Welcome Collection
Figure 1: Ambroise Paré, on the battlefield using a ligature for the artery of an amputated leg of a soldier. Wood engraving by Charles Maurand after E. Morin. Credit: Welcome Collection
Oranges and Lemons: The Age of Control
However, it was not until two hundred years after Paré’s accidental trial that the first documented planned or controlled clinical trial was performed. Dr James Lind, a Scottish naval surgeon, was concerned with the high mortality rate associated with scurvy amongst sailors at sea. In 1747, he undertook a detailed and systematic review of the available reports in the literature and, combined with his own first-hand experience, proposed six potential treatment options for inclusion in a comparative experiment. Twelve sailors affected by scurvy were selected for inclusion. All twelve were housed together and provided with a common diet. The group was divided into six subgroups, and each group given a scheduled test option over a 2-week period: cider; elixir vitriol (sulphuric acid and alcohol mix); vinegar; seawater; citrus (two oranges and one lemon); nutmeg. In Lind’s own summation “The consequence was, that the most sudden and visible good effects were perceived from the use of oranges and lemons; one of those who had taken them, being at the end of six days fit for duty … The other was the best recovered of any in his condition, and… was appointed to attend the rest of the sick”.4,5 Although not comparable to the citrus group, the men given cider had improved weakness and gums and those given the elixir of vitriol had clearer and better-conditioned mouths. No change in condition was noted for those who took seawater, vinegar, or nutmeg. The results of the trial were clear, the addition of citrus to the diet was deemed an effective treatment for scurvy. However, Lind hesitated to recommend the implementation of the citrus diet due to the monetary expense imposed on the Navy to deliver. It was nearly 50 years later and following the observational studies of fellow Scot Gilbert Blane on the effect of citrus on mortality rates amongst sailors in the 1780’s, before the British Navy introduced lemon juice (or the cheaper lime juice) as a compulsory part of the sailor’s diet.
Lind’s Treatise of 1753 contained not only the first description of a controlled trial showing that oranges and lemons were dramatically better than the other treatments for the disease but also the first systematic literature review and use thereof to shape trial design. The Treatise also clearly demonstrated the interaction between human benefit and the economic cost of implementation – a reality that remains evident in today’s healthcare decision-making processes.

Figure 2: A treatise on the scurvy. Containing an inquiry into the nature, causes, and cure, of that disease. Together with a critical and chronological view of what has been published on the subject. Credit: The James Lind Library.
Introducing the Placebo
Following the implementation of Lind’s work on scurvy, an increasing number of controlled comparative studies were undertaken to address various medical challenges within society. During this same period, we also see the first attempts to improve the validity of data sets generated. The word “placebo” first appeared in the medical literature in the early 1800’s and was defined as “an epithet given to any medicine more to please than benefit the patient.” However, it was 1863 before we see the first use of the placebo in a planned clinical trial.6 Austin Flint, an American physician, recorded the first study comparing a “dummy” remedy to an active ingredient in patients suffering from rheumatic fever. Flint treated thirteen hospital inmates who either had acute or sub-acute rheumatic fever with the placebo remedy – a diluted plant extract. He then compared outcomes with those observed for the active standard of care treatment – data for which had already been collected and documented. Flint demonstrated that there was no significant difference in the disease course in terms of disease duration, convalescence time, joints affected, or the emergence of complications, with the fever naturally subsiding over time and not because of the active treatment. Even though the placebo arm was conducted separately to the active arm, the study clearly marked a shift in scientific thinking towards the demonstration of a clear and positive benefit or effect of an active drug treatment compared to the natural progression of a disease. This quickly became the goal for subsequent investigations as the century progressed.
The Blinding Effect
The use of a control group and or placebo allowed informative comparison in research studies, however, there remained the element of bias (intentional or unintentional) in participant selection and data interpretation. Further improvement in the validity of datasets arrived in the 1940’s with the patulin trial. Patulin, an extract of Penicillium patulinum, was under investigation as a treatment for the common cold by scientists within the Medical Research Council (MRC) in the United Kingdom. A report in a British newspaper regarding the ongoing research led to some confusion, with the British government interpreting the report to suggest that a cure was ready for manufacture. To aid clarification of the situation, the MRC undertook a large multicentre controlled study.7 The MRC Patulin Clinical Trials Committee (1943), chaired by Sir Harold Himsworth, enrolled over one thousand British office and factory workers suffering from the common cold – a challenging endeavor in wartime Britain. The study was controlled by keeping the physician and the patient blind to treatment allocation thereby removing any bias in treatment and observation. This was performed using an alternation approach and strict segregation of allocating staff. The statisticians considered this an effective random allocation, and it was essentially the first double-blinded comparative trial documented. Unfortunately, when published, the trial showed no positive difference in treatment success between the groups. However, the design and conduct of the trial itself did highlight the importance of stakeholders (researchers, patients, funders, governments) working towards a common approach to a medical issue and, importantly, that the publication of negative or non-conclusive results from a rigorously controlled study could be as useful as positive observations in determining next steps. Double blinding has been considered an exemplary clinical trial approach since.
Randomization: the rise of the modern approach
Randomization as a concept has been around since the 1920’s but it was the MRC (UK), again, who first implemented its controlled use in a clinical trial. Chaired by Sir Geoffrey Marshall, the Streptomycin in Tuberculosis Trials Committee designed and operated the first randomized controlled trial of streptomycin for the treatment of pulmonary tuberculosis.8 The trial began in 1947 and was conducted using a randomization scheme designed by Sir Austin Bradford Hill, a key advantage of which was allocation concealment to all at the time of enrolment. Since the amount of streptomycin available from the US was limited at that time, it was deemed ethically acceptable for the control subjects to be untreated. Determination of whether a patient would be treated by streptomycin and bed rest or by bed rest alone was made by reference to a statistical series based on random sampling numbers drawn up for each sex at each centre. The details of the series were unknown to any of the investigators, study coordinators or donors, and were contained in a set of sealed envelopes, each bearing on the outside only the name of the hospital and a number. Additionally, the trial implemented objective measures such as interpretation of x-rays by experts also blinded to the treatment grouping, making the trial a model in design with systematic enrolment criteria and data collation. Hill’s randomization scheme, several years in the making, was first published in 1948 in the British Medical Journal and has since become the universal gold standard for clinical trial research - and very much the norm in today’s clinical trial landscape.
The Ethical Code
The evolution of the clinical trial continued throughout the latter half of the 20th century however, the focus changed from the design and interpretation of a study to consider the moral reasons for undertaking such research in the first place.
The origins of an ethical code are provided in the ancient Hippocratic oath – the oath of ethics historically taken by physicians. Within the oath, the principles of medical confidentiality and non-maleficence – first do no harm – are enshrined. These principles continue to guide and inform modern medical practice. Unfortunately, over the ensuing centuries the oath has not always been followed as it should and advances in ethics and the protection of human subjects have essentially evolved in response to human abuses.9
The Nuremberg Trials (1945-46) in which former Nazi leaders were indicted and tried as war criminals highlighted the extent of non-consensual human experimentation performed by Nazi physicians on prisoners. Pharmaceutical testing, war injury simulation, reproductive sterilization, and many other cruelties were documented. The outcome of these recorded abuses was the introduction of the Nuremberg Code in 1947, also known as the International Code of Medical Ethics. The code detailed the minimum requirements for the protection of humans participating in clinical trials – essentially that consent should be voluntary and that the potential benefit must outweigh any potential risk.
Although widely accepted as good practice, the code was not enforceable and over the next 2 decades unethical practices in clinical trials continued. One of the most notable was the Tuskegee Study of Untreated Syphilis in the Negro Male, initiated in 1932 to investigate options for the treatment of syphilis.10 A United States government-funded study, it ran for 40 years with no regard to the introduction of the Nuremberg code or the introduction of penicillin as the standard of care treatment for syphilis in the same year. The systematic exploitation of participants and withholding of a readily available treatment in the cause of science consequently led to the deaths and infections of participants, their partners, and their children. The study subsequently became a symbol for racism in medicine, misconduct in human research, the arrogance of physicians, and government abuse. In 1997, in a White House ceremony, President Bill Clinton apologized for the study, saying that the legacy “has reached far and deep, in ways that hurt our progress and divide our nation”. It was, indeed, a first-class example of the need for the provision of protections for research participants and the priority of human health over the scientific question.
In 1964, in a bid to develop the principles detailed in the Nuremberg code, the World Medical Association published the Declaration of Helsinki – an international set of guidelines encompassing research ethics for research as part of medical care and for non-therapeutic research approaches. Since then, the Declaration has undergone various revisions and additions to reflect advances and discussions in ethical conduct. For example, the 1975 revision introduced the concept of oversight and the establishment of review boards or committees – now standard practice in most countries. The Declaration guidelines have been adopted and developed by many governments across the world as the basis for national and international laws and regulations that govern human research. As such, seeking ethical approval from a committee is a legal formality required prior to the initiation of a clinical study in most democratic countries.
In parallel, clinical trials themselves have become embodied in regulation as governments and regulatory bodies recognized the need to control and monitor new medical therapies before they become mainstream in the population. Agencies such as the Food and Drug Administration (FDA) in the US, the European Medicines Agency (EMA) and the Medicines and Healthcare product Regulatory Agency (MHRA) in the UK oversee the licensing of new drugs and therapies for much of the world’s population. With legislation demanding accountability and evidence for all aspects of the development and manufacturing of a new drug, the need for testing in controlled clinical trials has never been higher. This interlinking of ethical conduct, clinical trial regulation, and legislation will continue to evolve as innovative technologies and scientific disciplines come online.
The Future
Throughout history, scientific challenge and the advancements developed in response to the challenge have laid the foundation for robust ethical research approaches. Evidence-based practices, standardized procedures, and the implementation of processes for the protection of participants, are par the course for today’s clinical trial. However, as we move forward, new scientific challenges will arise and the design and conduct of clinical trials must also rise to meet these challenges.
Today’s challenges such as: the collection and use of big data; trial design for pharmaceutical marketing; public health initiatives; and the soaring costs – both monetary and time –are already shaping the next evolution in clinical trial design and implementation.
Digital technology and its use in clinical research, has been under much discussion in recent years. Obtaining, manipulating, and storing large data sets has pushed the limits of concern around privacy, access, and respect for the participant. Consequently, changes in the regulations for obtaining and storing data have been updated around the globe. However, digital technology is also transforming the clinical trial process itself.11 In 2016, the US enacted the 21st Century Cures Act, designed to help accelerate medical product development and innovation. The real world data and real world evidence that the act encourages has led to a willingness to include data captured from mobile devices, videoconferencing, web-based tools, and biosensors in various aspects of clinical research. Essentially bringing the trial closer to the patient, via technologies used in everyday life. The concept of this decentralized or virtual trial approach has been growing slowly ever since and has accelerated significantly following the recent SARS-CoV-2 pandemic, where restrictions in face-to-face approaches and increased hospital burden forced researchers to investigate alternative approaches to the standard site-based, centralized research format. One of the concerns for decentralized approaches is ensuring that the quality of the trial and the integrity of the data that is collected are not compromised – two of the main tenets of the randomized controlled trial. As more technologies, and the novel trial methods they power, are proven to be effective (and approved by regulators) the more likely the dependence on site-based trials will lessen, and the focus on decentralized models that enable remote procedures and visits will increase. It remains to be seen how this hybrid approach to clinical trials will play out but as we move towards this new era, researchers should remember and consider the long history that has led to this point.
Outsourcing Clinical Trial Support
If you are looking to outsource support for your clinical trials, REPROCELL provides a range of services to complement your clinical strategy. Whether you require help with logistics, tissue access, or biospecimen processing, you can find a full range of support available on our website. Alternatively, you can contact our team to arrange a custom solution for your research.
References
- Bhatt A. Evolution of clinical research: a history before and beyond James Lind. Perspect Clin Res. 1:1 pp 6–10 (2010).
- Collier R. Legumes, lemons, and streptomycin: A short history of the clinical trial. Can Med Assoc J. 6;180(1) pp 23–4 (2009).
- Markatos K et al. G. Ambroise Paré (1510-1590) and his innovative work on the treatment of war injuries. Surg Innov. 25(2) (2018).
- Lind J. A Treatise on the Scurvy in Three Parts [Internet]. London (1772).
- Bartholomew M. James Lind ’s Treastis of the Scurvy. Postgrad Med J. 2002;78 (1753).
- Flint A. A contribution toward the natural history of articular rheumatism; consisting of a report of thirteen cases treated solely with palliative measures. American Journal of the Medical Sciences. 1863; 46 pp 17–36.
- Medical Research Council. Clinical trial of patulin in the common cold: report of the patulin clinical trials committee. The Lancet 16;244(6316) pp 373–5 (1944).
- Medical Research Council. Streptomycin treatment of pulmonary tuberculosis. Br Med J. 2:769–82 (1948).
- Metcalf J. Ethics Codes: History, Context, and Challenges. (2014).
- Baker SM, Brawley OW, Marks LS. Effects of untreated syphilis in the negro male, 1932 to 1972: A closure comes to the Tuskegee study. Urology 2005 65:6 pp 1259–62 (2004).
- Dorsey ER, Kluger B, Lipset CH. The New Normal in Clinical Trials: Decentralized Studies. Ann Neurol. 11;88(5) pp 863–6 (2020).
Scientific Observations and Developments Throughout the Ages Contributing to the Evolution of the Clinical Trial
460-370 BC
Hippocrates championed the art of clinical inspection, observation, and documentation. Introduced clinical records and recognised cleanliness in the management of wounds.
450-350 BC
Hippocratic Corpus.
130-200 AD
Galen of Pergamum, physician to the Roman Emperor Marcus Aurelius, first to implement an animal study to understand disease.
1061
Ben Cao Tu Jing documents an observational trial of ginseng in the ‘Atlas of Materia Medica’.
1537
Ambroise Paré performed the first clinical trial of a novel therapy.
1662
The foundations of demography with the publication of Graunt’s ‘Natural and Political Observations Mentioned in a Following Index, and Made Upon the Bills of Mortality’.
1667
The first blood transfusions in humans by Richard Lower and Edmond King.
1660-70’s
Invention of the microscope by Antony van Leewenhoek and the first visual documentation of microbes.
1723-27
Introduction of the concept of mortality as a critical endpoint by James Jurin.
1753
James Lind published ‘A Treatise on Scurvy’, the first controlled trial and systematic literature review to aid design and implementation
1780
Gilbert Blane published ‘On the most effective means for preserving the health of seamen, particularly in the Royal Navy’. A large-scale study of mortality and illness in response to treatment leading to policy change
1786
Caleb Parry undertook the first crossover trial in patients to test the laxative properties of two types of rhubarb root.
1796
Edward Jenner and the seminal vaccination programme for the prevention of smallpox.
1809
Alexander Lesassier Hamilton performed the first randomised approach to a trial testing the effectiveness of blood letting.
1863
Austin Flint demonstrates the use of a placebo in a trial to show a positive treatment benefit.
1943
MRC (UK) Patulin Trial demonstrated the first use of double blinding in a trial for the treatment of the common cold.
1947
Introduction of the Nuremberg code.
1948
MRC (UK) Streptomycin in Tuberculosis Trial demonstrated the first use of a randomised controlled approach to trial design and data interpretation.
1951
Austin Hill and Richard Doll initiate the British Doctor's Study, one of the first large scale, long term, observational studies of disease risk associated with smoking.
1962
Thalidomide scandal and the introduction of strict regulation of human trials with respect to patient safety.
1964
Declaration of Helsinki published by WHO.
1975
Adaptation of the Helsinki Declaration to Establish ethical review committees to consider safety and benefit of trials.
1970-80's
Clinical Research Organisations established as specialist in conducting trials to the highest standards of quality, reliability, and transparency. The rise of the Central Laboratory.
2016
The 21st century Cures Act enacted in the US.
2018
The General data protection Regulation (GDPR) introduced across Europe.
2020
SARS-CoV-2 pandemic and vaccine development programmes boost decentralised clinical trial approaches.