Progressive and innovative Pharmaceutical and Biotechnology companies ask themselves several key questions when it comes to Drug Discovery and Development:
• How and where can we be research smarter?
• How can we reduce uncertainty?
• How can we better predict efficacy and safety?
• How can we reduce costs?
One route to making the drug discovery process smarter and more efficient is to use human tissue at one or more stages in the development process.
Why use Human Tissue in drug discovery?
Recent analysis and review of the pharmaceutical industry suggests that it is still difficult to translate potential drug candidates into safe, effective medicines. Less than 10% of candidate drugs succeed in hurdling the many challenges of the pre-clinical and clinical phases to attain final approval for use in the clinic.
Although the end target for a new medicine is man, the methods used to identify, develop, and validate a potential drug candidate have traditionally relied on non-human assays. The ability of such methods to predict efficacy and safety can be unreliable and not reflective of the human response.
The use of human tissue derived data to validate or replace non-human methods can facilitate better interpretation of the data and enhance the decision-making process. This is important when one considers that the average cost of bringing a new drug to market is estimated to be around £2 billion.
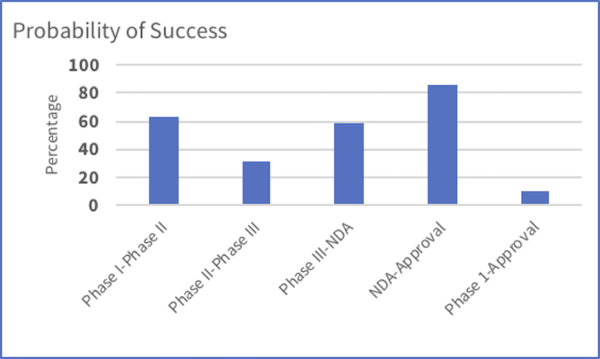
Figure 1: Probability of clinical success decreases significantly during Phase 1 Approval [1]
When is Human Tissue used in research?
Human tissues, whether surgically resected and surplus to diagnostic requirements, donated post-mortem or donated viably, are available in a variety of formats for use in different applications. Human tissue assays can therefore be applied to all stages of the drug development process, from target identification to clinical trial.
Since most new drugs fail to reach the clinic, it is economically beneficial to identify failure as early in the process as possible and to re-direct resources to drug candidates with better potential: the "fail early, fail cheap" approach.
Advantages of using a Human Tissue Provider
Obtaining and working with human tissue can often be challenging. Legal, ethical, logistical, and practical issues all need to be addressed to enable the procurement of ethically-consented, good-quality tissues at the exact time of need.
These obstacles to accessing tissue can force researchers to continue with the more traditional routes of non-human assays for drug discovery and development. Procuring the expert services of a tissue provider is one solution to overcoming these obstacles, enabling researchers to:
- Reduce Logistical Challenges
- Assistance with Regulatory and Ethical Processes
- Access to Global Tissue Sourcing
- Reduce use of Animal Tissue and Models
Human fresh tissue services at REPROCELL
REPROCELL’s ethically-sourced human tissue network underpins our core business model: the provision of high-quality human tissues and assay services to support drug discovery and development.
Through a combination of our extensive Biorepository (BioServe), Stem Cell Services (Stemgent) and Predictive Drug Discovery Services (Biopta), we provide the Pharmaceutical and Biotech industry with a comprehensive solution to their human tissue research needs.
With our help, human tissue research can be a reliable and valued component of the drug discovery process. Visit the REPROCELL website to explore our range of research tools and services.
References
- Thomas et al. Clinical Development Success Rates 2006-2015. Bio (2016)