Blog

Enhancing Experimental Precision with the Alvetex® Advanced Culture System
Discover how Alvetex® Advanced enhances experimental precision with its flexible and functional 3D culture system for creating biologically accurate human skin models.
04 December 2025

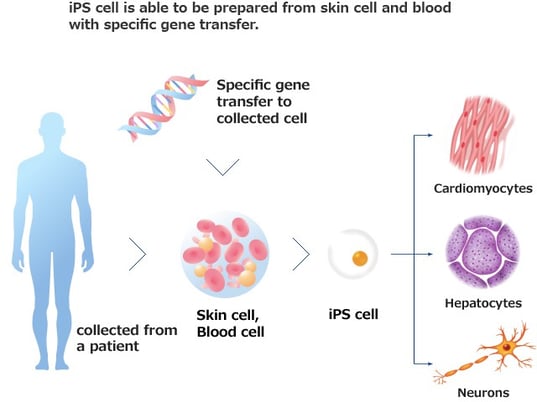
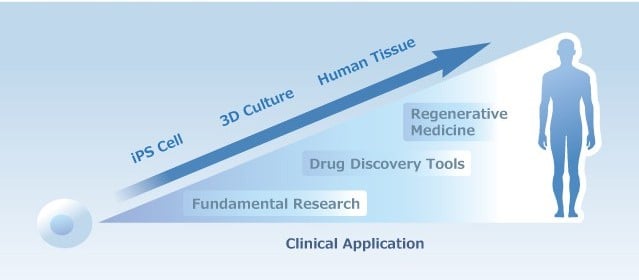
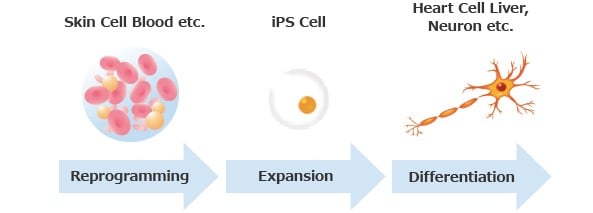
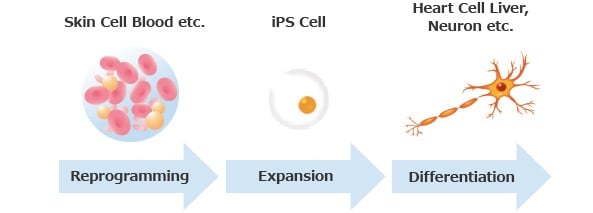
iPSC-, MSC- and iMSC-Derived Exosome Therapeutics: The Cell-Free Future of Regenerative Medicine
Explore the future of regenerative medicine with iPSC-, MSC-, and iMSC-derived exosome therapeutics, focusing on their advantages, challenges, and clinical potential.
19 November 2025

Using Ex Vivo Skin Explants to Study Local Immune Responses in Psoriasis and Atopic Dermatitis
Explore the use of ex vivo skin explants to study local immune responses in chronic inflammatory skin diseases like psoriasis and atopic dermatitis.
17 November 2025
.jpg?width=1000&height=562&name=Untitled%20design%20(5).jpg)
The Enduring Value of Organ Baths in Modern Drug Discovery
Discover the enduring value of organ baths in drug discovery and their critical role in ensuring the efficacy and safety of new therapies.
06 November 2025
Upcoming Events
Conferences we will be attending, and webinars hosted by us
Meet us at REPROCELL booth and learn about our CLINICAL STEM CELL SERVICES at the ISSCR International Symposium 2025 / 11-12 December / Boston MA, USA
Come meet us on 9-12 February 2026 / San Diego, California, USA. Venue: Convention Center San Diego.
Corporate News
REPROCELL announces the launch of new hypoimmune hiPSC lines using advanced gene-editing technologies, enhancing precision and efficiency in regenerative medicine and research applications.
07 November 2025
REPROCELL USA Receives Funding from the Maryland Stem Cell Research Fund (MSCRF)
REPROCELL USA receives Maryland Stem Cell Research Fund grant to develop GMP-grade CDMO and iMSC capabilities, enhancing their regenerative medicine and stem cell therapy manufacturing infrastructure.
22 October 2025
REPROCELL Launches Next-Generation System for 3D Bioengineered Tissues
REPROCELL launches Alvetex® Advanced, enhancing 3D cell culture and bioengineered tissue models with improved flexibility and assay compatibility for pharmaceuticals, cosmetics, and medical device testing.
21 October 2025