Wheat Germ Agglutinin Staining of 3D Cultures Grown on Alvetex® Scaffold and Alvetex Strata
● Download this protocol as a PDF (2 MB)
1. Introduction
Wheat Germ Agglutinin (WGA) is a well-known lectin originally noted for its differential ability to agglutinate cancerous versus normal cells[1] and later identified as a plasma membrane staining agent of both animal[2] and bacterial[3] cells, thanks to its binding of N-acetyl-D-glucosamine and N-acetylneuraminic acid residues[4] on plasma membrane glycoproteins. WGA is now commonly used to visualise cell boundaries and as a double-stain to ascertain membrane proteins localisation.
This document contains is an example protocol for the WGA staining of cryosections of cultures of the hepatocarcinoma cell line HepG2[5] in both Alvetex Scaffold and Alvetex Strata. It is recommended that for any dye used, the original manufacturers’ instructions for fixing, blocking and incubating methods be followed. Imaging of the specimens will also need to be optimised according to the specifications of the microscope used.
2. Preparation for 3D cell culture on Alvetex
- HepG2 cells were routinely maintained in T-75 flasks
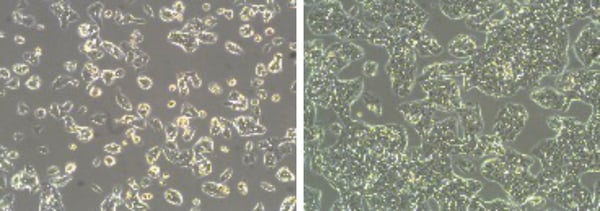
Figure 1: Phase contrast micrographs of HepG2 cells grown in conventional 2D culture plates. Images show cells at low (left) and high (right) confluency. Scale bars: 100 µm.
- Complete media consisted of: MEM media (ThermoFisher 21575097) supplemented with 10 % v/v FBS and 100 U/mL Penicillin/Streptomycin. 21575097 already contains 2 mg/mL L-glutamine.
- Cells were harvested by trypsinisation and centrifuged for 5 minutes (1000 rpm). The supernatant was discarded and the cell pellet was re-suspended in an appropriate volume of media for cell counting by Trypan Blue.
- Cells were re-suspended at a concentration of 2 × 106 cells/mL for seeding.
- Alvetex Scaffold and Alvetex Strata 6-well inserts were prepared for seeding by dipping in 70 % ethanol and washed twice with 7 mL of media per well.
- Media was added to each well to obtain an ‘above and below’ regime, i.e. enough media to submerge the Alvetex disc but not to go over the insert windows.
- 500 μL of the cell suspension was added drop-wise all over the disc, which was equivalent to 1 × 106 cells per well.
- The plate was incubated 30 min at room temperature to allow the cells to settle onto the disc.
- Plates were returned to 37°C with 5% CO2 to complete cell attachment overnight.
- The following day, media was added to each well taking care not to dislodge cells, up to an ‘interconnected’ regime, i.e. enough media to submerge the Alvetex disc and go over the insert windows.
- Plates were re-incubated and maintained by complete media exchange after every 2-3 days.
3. Preparation of samples for fixation, embedding and cryosectioning
- Samples were processed by fixation, optimum cutting temperature (OCT) medium embedding and cryosectioning according to method 3 of the protocol Processing Alvetex Scaffold 3D Cultures for Cryosectioning (available at Alvetex Protocols).
- Alvetex cultures were removed from their inserts and washed twice in phosphate-buffered saline (PBS).
- Cultures were transferred to a 4% solution of paraformaldehyde (PFA) in PBS and fixed overnight at 4 °C.
- Excess water was removed gradually in two baths of sucrose, 15 % followed by 30 % (overnight at 4 °C in each bath).
- Discs were cut into smaller pieces and transferred to plastic embedding moulds containing OCT mounting medium and allowed to soak for a few minutes at room temperature.
- Embedding moulds were placed on dry ice and left to freeze gradually over 30 min.
- Samples were stored at –80 °C until sectioning following the cryostat manufacturers’ instructions.
- Sections were adhered to negatively-charged microscope slides and air-dried to promote attachment before processing to WGA staining.
4. Preparation of samples for WGA staining
Note: As WGA specifically targets the plasma membrane compartment, care must be taken not to permeabilise the samples before staining. When double-staining with a dye or antibody for which permeabilisation is necessary, perform WGA staining first and then permeabilise the sample, using a low concentration of detergent and a short exposure time whenever possible.
- To ensure that an appropriate excess of wash solution was used, slides were placed in a Coplin jar and washed twice in PBS.
- Slides were transferred to a horizontal rack and 200 μL of fluorescein-labelled WGA (FL-1021, Vector laboratories) diluted to 5 µg / mL in PBS was added to each slide.
- Slides were left for 10 min at room temperature protected from light.
- Slides were returned to the Coplin jar and washed in PBS twice.
- Slides were returned to the horizontal rack and one drop of hard mounting medium containing DAPI (H1500, Vector Laboratories) was added to each slide.
- Slides were left for 15 min at room temperature protected from light to initiate mounting, after which they were stored at 4 °C protected from light until imaging.
5. Example pictures
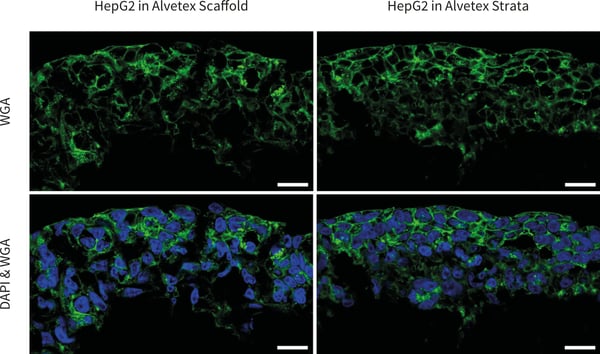
Figure 2. Confocal microscopy images of HepG2 cells cultured in 6-well inserts Alvetex Scaffold (left) and Alvetex Strata (right) and stained with WGA and DAPI. Note that the cell boundaries are clearly delineated. Pictures taken using a ZEISS 880 confocal microscope, scale bars: 20 µm.
6. References
- Aub JC, Sanford BH, and Cote M N (1965). Studies on Reactivity of Tumor and Normal Cells to a Wheat Germ Agglutinin. Proceedings of the National Academy of Sciences of the United States of America 54(2), 396-399.
- Gonatas NK, and Avrameas S (1973). Detection of Plasma-Membrane Carbohydrates with Lectin Peroxidase Conjugates. Journal of Cell Biology 59(2), 436-443.
- Lotan R, Sharon N, and Mirelman D. (1975). Interaction of Wheat-Germ Agglutinin with Bacterial-Cells and Cell-Wall Polymers. European Journal of Biochemistry 55(1), 257-262.
- Bhavanandan VP, and Katlic AW (1979). Interaction of Wheat-Germ Agglutinin with Sialoglycoproteins — Role of Sialic-Acid. Journal of Biological Chemistry 254(10), 4000-4008.
- Knowles BB, Howe CC, and Aden DP (1980). Human Hepatocellular-Carcinoma Cell-Lines Secrete the Major Plasma-Proteins and Hepatitis-B Surface-Antigen. Science 209(4455), 497- 499.