Collagen I Coating of Alvetex® Scaffold
● Download this protocol as a PDF (0.8 MB)
1. Introduction
The following protocol outlines how to coat Alvetex Scaffold membranes with a thin layer of collagen I in order to facilitate and enhance cell attachment and migration within the scaffold. Example data shown herein was obtained using this protocol to grow HepG2 hepatocytes on collagen I coated Alvetex Scaffold for 7 days in 6-well inserts (AVP004) in 6-well plate format.
2. Method
- Prepare Alvetex Scaffold for coating by first treating with 70% ethanol followed by two PBS washes as described in the relevant product information leaflet. Leave Alvetex Scaffold in the second PBS wash until ready to apply the collagen solution.
- Dilute rat tail collagen I (BD Biosciences (Corning), 354236) to a concentration of 0.8 mg/mL using cell culture grade water. Handle the reagents on ice, using pre-chilled pipette tips to perform the dilution and subsequent application onto Alvetex Scaffold.
- Aspirate the second PBS wash from Alvetex Scaffold disc and carefully pipette 500 μL of the diluted collagen solution onto each disc. Replace plate lids and leave to stand for 1 hour at room temperature.
- Remove excess fluid from Alvetex Scaffold in well insert format by gently tapping the plate or Petri dish on the worktop. Check that no residual fluid is hanging from the base of the well inserts. Aspirate to remove any residual coating agent from the bottom of the wells. If using Alvetex Scaffold in 12-well plate format, tilt the plate and gently aspirate any excess fluid from the edge of the wells.
- Prepare cells for seeding in the appropriate culture media and seed directly on the wet collagen coated Alvetex Scaffold membrane in the volumes relevant for Alvetex Scaffold product format. Allow the cells to settle for 30-90 minutes in an incubator (5 % CO2, 37 °C) before flooding with media.
3. Example: growth of HepG2 hepatocyte cell line in collagen I coated Alvetex Scaffold
3.1. Cell Culture details
HepG2 cells (ATCC, HB-8065) were routinely maintained in T-75 flasks. HepG2 complete media consisted of: MEM media (Gibco (Thermo Fisher), 21090) supplemented with 10 % v/v FBS, 2 mM L-glutamine and 100 U/mL Penicillin/ Streptomycin. Alvetex Scaffold 6-well inserts (AVP004) in 6-well plates, were coated in collagen I as described above.
Cells were seeded at a density of 1 × 106 cells in 150 μL media suspension per disc and were left to settle for 90 minutes in an incubator (5 % CO2, 37 °C). Media was carefully added to each sample (9 mL per well). Cultures were maintained for 7 days, with media changes on 2, 3, and 6.
3.2. Results
Pre-coating of Alvetex Scaffold discs with collagen I resulted in enhanced infiltration of HepG2 cells into the scaffold compared with control cultures in untreated Alvetex Scaffold. Cells were seen to reside deep within the scaffold after 7 days of growth in treated discs, while cells grown in untreated Alvetex Scaffold occupied only the upper half of the scaffold. These findings indicate that pre-treatment of Alvetex Scaffold with extracellular matrix products is able to enhance the attachment and growth of appropriate cell types into the 3D structure.
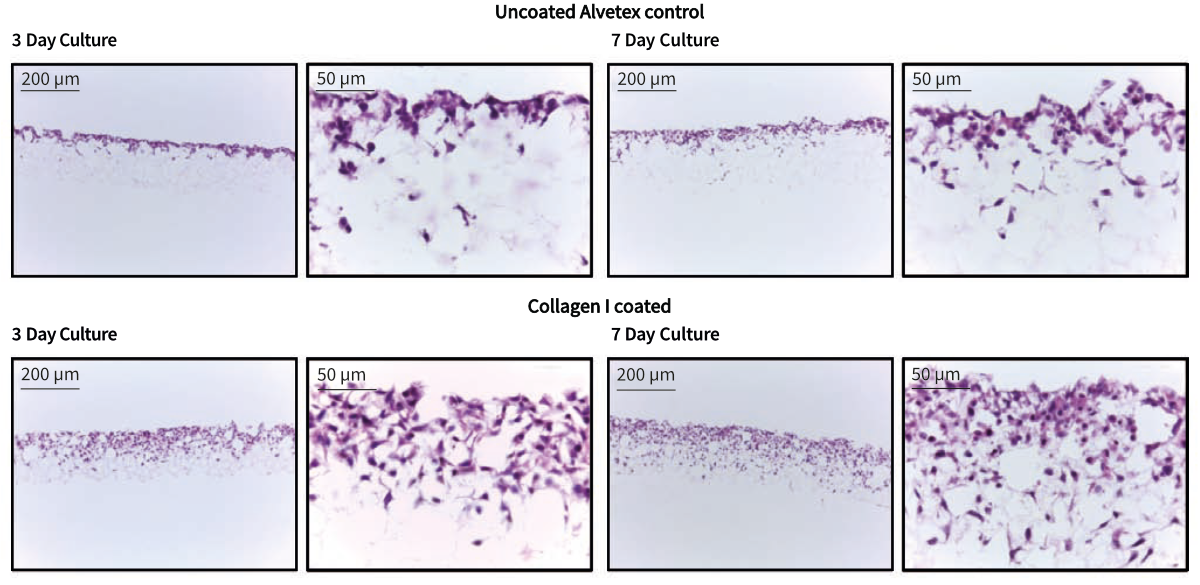
Figure 1. Brightfield micrographs at low (10×) and high (40×) magnification showing HepG2 cells cultured for up to 7 days on 22 mm diameter Alvetex discs presented in 6-well insert (AVP004) in 6-well plate format. Cells were fixed, sectioned and counterstained with haematoxylin and eosin.