Alvetex® Scaffold 384 Well Plate: Instructions For Use and Sample Application
● Download this protocol as a PDF (1.2 MB)
REPROCELL’s Alvetex Scaffold 384 well plate format is comprised of a black plate, clear plastic base, with Alvetex Scaffold at the bottom of each well. The Alvetex Scaffold has been heat welded to the base of the wells. Cells are exposed to culture medium from above only, and therefore predominantly reside in the top portion of scaffold. As with 2D culture, if using serum-free medium, the use of coating reagents to enhance cell attachment can be considered.
Figure 1. Alvetex Scaffold in 384 well plate format.
1. Notes before starting
- Hydration of the Alvetex Scaffold is essential by placing 100 µL of 70 % ethanol into each experimental well.
- Each experimental well was washed with 100 µL of 1 × PBS and then 100 µL of culture media.
- Final media wash was aspirated and 50 µL of fresh media was placed into each experimental well and placed in the incubator at 37 °C / 5 % CO2 until ready for cell seeding.
2. Optimisation of cell seeding for Alvetex Scaffold 384 well plate format
- Use standard 2D detachment methods and count cells using Trypan Blue and haemocytometer.
- PC-3 cells were seeded at the following cell density for evaluation: 1000, 2000, 5000 and 10000 cells per well in a final volume of 50 µL. Cells were grown for periods of 1, 4 and 7 days and their growth and viability assessed by MTS assay.
3. Example application: MTS assay protocol
- 60 µL of MTS reagent was added to each sample well (50 µL culture media and 10 µL of MTS reagent).
- 384 well plates were incubated at 37 °C / 5 % CO2 for 60 minutes in the dark, and the absorbance measured at 490 nm.
- After 24 hours of culture, a linear MTS assay response with increasing cell number was observed (Fig. 2).
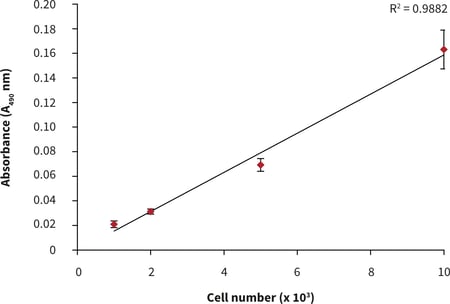
Figure 2. Cell number standard curve for PC-3 cells cultured on 384 well plates. n = 12 ± SD.
- In some cases, absorbances may decrease towards the centre of the plate due to edge effects. This phenomenon is common with high throughput plates such as the 384 well and 96-well plate formats.
- Given that pipetting errors may also occur when manually removing the product from a 384 well plate it is recommended that the 384 well plate is used on an automated liquid handling platform due to the small volumes used.
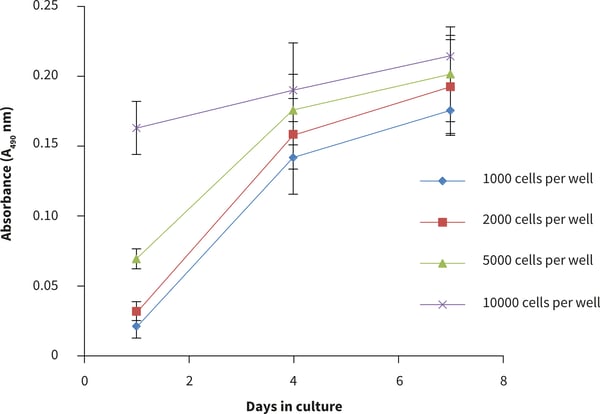
Figure 3. Cell number standard curves for the PC-3 cells using the MTS cell viability assay. PC-3 cells were seeded at a range of densities onto Alvetex Scaffold 384 well plate format and cultured for up to 7 days hours prior to analysis. Data represent mean ± SD, n = 12.
- The viability curve shows similar growth patterns for 1000, 2000 and 5000 cells seeded per well. At a cell seeding density of 10 000 cells per well, slower growth was observed compared to the lower cell seeding numbers. In this case the number of cells per well was exceeded.
- From these observations, the optimal growth conditions for PC-3 cells on the 384 well plate is recommended to be between 1000-5000 cells per well for up to four days culture.
4. Example data derived from Alvetex Scaffold 384 well plates
For drug assessment studies in the 384 well plates, PC-3 cells (2,000 cells per well) were cultured for 24 hours prior to exposure to docetaxel (0-100 nM) and doxorubicin (0-10 µM) for 72 hours. Cell viability was evaluated using the MTS assay and the percentage viability calculated against a non-drug treated control
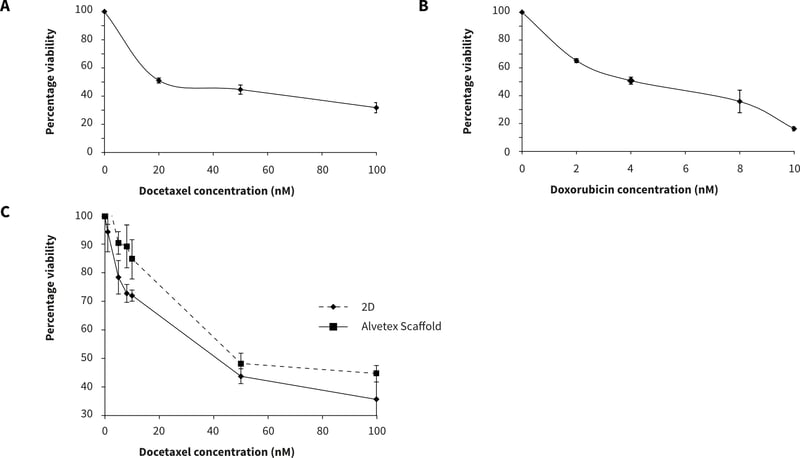
Figure 4. PC-3 response to docetaxel (A) and doxorubicin (B) in Alvetex Scaffold 384 well plate format (2000 cells per well) and (C) PC-3 (15000 cells per well) in response to docetaxel in Alvetex Scaffold 96 well plate format. n = 12 ± SEM (384 well plate), n = 6 ± SD (96-well plate).
Alvetex Scaffold 384 well plate format can provide a unique platform for the screening and development of specific chemotherpapeutic drugs. Responses observed in 384- and 96-well plate formats are consistent: for example the PC-3 response to docetaxel in Alvetex Scaffold 384 well plate format is comparable to the response obtained in the 96-well plate format. However, when making direct comparisons, it is also important to consider the differences in the numbers of cells seeded per well.