Alvetex Strata Application Note 02
Propagation of Human Pluripotent Stem Cells in Three-Dimensional Culture Using Alvetex Strata
Download this application note as a PDF (14.15 MB)
Overview
We have developed a strategy that enables the contin-uous propagation and maintenance of cells in three-dimensional (3D) culture. The technology relies on culturing cells continuously on the surface of Alvetex Strata, a highly porous membrane presented in a well insert format. As proof of concept, we demonstrate the propagation of human pluripotent stem cells in 3D over many passages. We show how these stem cells adapt to their surroundings and acquire a more 3D shape. Such cells appear to become ‘primed’ for growth in 3D and consequently show marked improvements in their developmental potential when subsequently grown in a range of established 3D cell differentiation models. This application note demonstrates how using Alvetex Strata offers a new approach to cultivating cells continuously in 3D to achieve notable improvements in cell growth and function. It can be used to routinely maintain proliferative cells with a 3D structural phenotype in preparation for their use in downstream assays that involve 3D cell growth.
Introduction
The majority of assays that involve 3D cell culture technology most frequently rely on the use of cells that have previously been grown in conventional plasticware. Cells are that cultured and expanded in number, are routinely maintained in polystyrene vessels such as flasks and Petri dishes. Many of the popular cell lines used in biomedicine for research and discovery are adherent to plastic and grow as flat monolayers. It is well known that cells adapt to this artificial environment and become accustomed to such growth by altering their behavior. These changes include alteration to cell proliferation rate, remodeling of the cytoskeleton, differences in gene and protein expression, and changes to cellular differentiation and function. There is good evidence to suggest that cells have therefore become adapted to growth on two-dimensional (2D) flat substrates and as a result their phenotype has changed. For example, tissue-specific architecture, mechanical and biochemical cues and cell-cell interactions are altered or lost under the simplified conditions of 2D cell culture (Bissell et al. 2003; Cukierman et al. 2001, 2002; Nelson & Bissell, 2006).
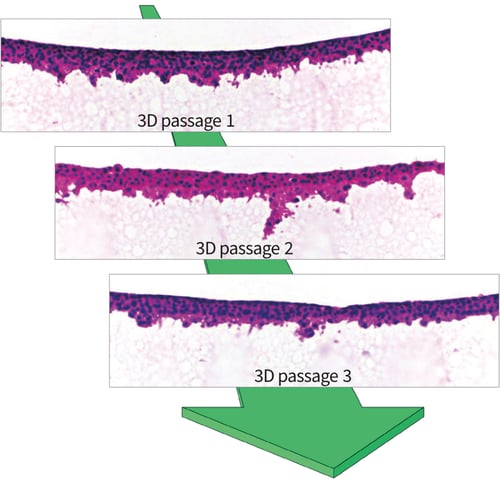
Above: Histological images of H&E stained TERA2.cl.SP12 cells that have been 3D passaged every 4 days on Alvetex Strata. Unlike with 2D cell growth on the flat substrate of traditional plastic-ware, cells grown on Alvetex Strata maintain 3D morphology and function throughout repeated 3D passaging.
Three-dimensional cell cultures mimic to a certain degree the in vivo situation by preserving the 3D integrity of individual cells, by allowing cell aggregation and direct signaling, and enabling cells to create their own niche microenvironment in conjunction with their extracellular matrix. There is good evidence to show that 3D culture models can be used to study human tissue physiology in health and disease where tissue-like function and homeostasis could be maintained (Schmeichel & Bissell, 2003). It is common practice however to expand populations of cells in 2D culture and then seed such cells into various types of 3D culture model to simulate a more natural growth environment, and to facilitate and enhance cell structure and function. In such circum-stances, cells are simultaneously: (a.) adapting to their new surroundings (2D to 3D growth transition); and (b.) being challenged and/or examined as part of the 3D cell culture assay (for example, a drug cytotoxicity test). Given that cells are exposed to two variables that are likely to influence their behavior, it is sometimes difficult to decipher the precise outcome of the experiment in a reproducible and robust manner.
Cells already growing within a 3D environment possess a more natural 3D phenotype. Such cells are therefore likely to more readily adapt to a 3D culture model that is designed to maintain such structure (without the need for 2D to 3D transition). Indeed, in many instances, cells from primary sources direct from tissues and explanted into 3D culture models, often show greater cell viability and functional characteristics compared to their counterparts seeded onto 2D conventional plasticware (Schutte et al. 2011). There are many possible explanations for such differences but conserving the native 3D structure of the cell is no doubt an important factor.
Maintenance of the 3D phenotype of a cell is therefore critically important and follows the fundamental principle ‘from structure comes function’. Reinnervate specializes in the development of enabling technology that follows this principle to enhance the growth, differentiation and function of cultured cells and tissues. Our products are designed to enhance the physiological relevance of in vitro models, provide researchers with new opportunities to culturing and studying cells and tissues in the laboratory, and improve methods for sample handling and analysis of cell-based assays. Alvetex Scaffold is our market leading product that is used for the development of a range of novel 3D culture applications for a diverse range of cell and tissue types.
In this article we demonstrate how Alvetex Strata, our new form of porous material, can be used to support the growth of human pluripotent stem cells on the surface of the membrane. Culturing cells in 3D using Alvetex Strata technology will lead to new opportunities to readily access viable cells and enable their continual propagation thus maintaining their 3D structural phenotype.
Methods to maintain human pluripotent stem cells in culture have been developed over many years and have involved many alternative approaches. Pluripotent stem cells are unique in that they have the ability to self-renew and retain the capacity to differentiate into all tissue types. Stem cells reside in a niche microenvironment that comprises physiochemical and biological cues that control cell proliferation and differentiation. In culture, stem cells have a propensity to spontaneously differen-tiate and significant research has gone into developing ways to maintain the stem cell phenotype whilst enabling expansion of the cell population and prevention of cell differentiation. Most often embryonic stem cells are grown in the presence of embryonic fibroblast feeder cells but more recently specialist media have been developed enabling feeder cell independent approaches. The majority of such stem cell culture is however performed in conventional 2D plasticware, most often to generate sufficient stem cell numbers for subsequent cell dif-ferentiation studies. Here we show how Alvetex Strata can be used to propagate and maintain pluripotent stem cells in 3D and show evidence of how this can also influence their developmental potential.
Results
At first glance, the structure of Alvetex Strata appears similar to Alvetex Scaffold: both products are made from the same crossed-linked polystyrene; both membranes are 200 microns thick; both are presented in our custom 6-well and 12-well inserts; and both come blister packed, sterile, ready for use. The difference however between these two materials concerns their fine structure and architecture. Both materials are highly porous and comprised of voids and interconnecting pores: in Alvetex Strata the voids and pores are significantly smaller (average 13 and 5 micron diameter, respectively) compared to those in Alvetex Scaffold (see Figure 1).
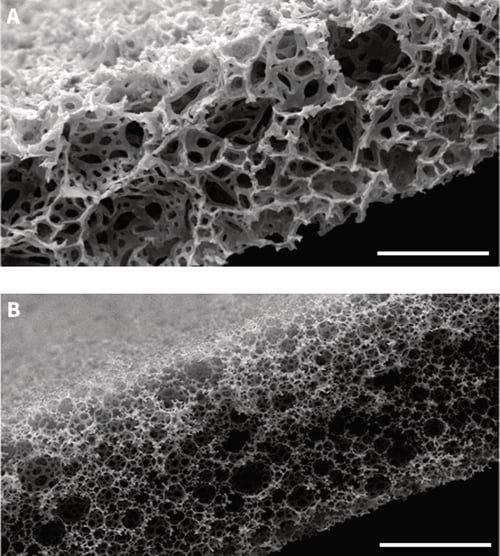
Figure 1: Scanning electron micrographs showing the structure of Alvetex Scaffold (A) and Alvetex Strata (B) porous polystyrene membranes in transverse section. Note that the size of the voids is significantly smaller in Alvetex Strata compared to Alvetex Scaffold. Scale bar: 100 microns.
The concept of propagating pluripotent stem cells on Alvetex Strata was initially developed using the human embryonal carcinoma lineage, TERA2.cl.SP12. Embryonal carcinoma cells are the malignant counterparts of embryonic stem cells and serve as useful research tools for studying certain aspects of embryogenesis and development (Przyborski et al. 2004). Moreover, TERA2.cl.SP12 are relatively inexpensive to work with and this supports the ability to test multiple growth conditions and optimize methodology. Having developed conditions to propagate TERA2.cl.SP12 cells on Alvetex Strata, the growth and developmental potential of the cells was characterized using standard techniques. Similar culture methods were then applied to demonstrate the propagation of human embryonic stem cells (clone RC-10, Roslin Cellab) on Alvetex Strata.
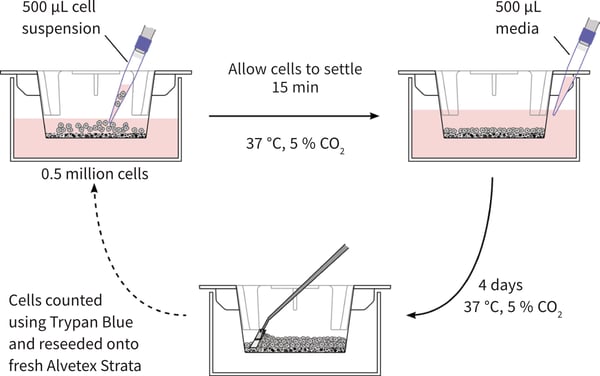
Figure 2: Schematic illustrating the method of propagating pluripotent stem cells in 3D using Alvetex Strata. Strata membranes are initially ethanol treated to render them hydrophilic and suspended in 6-well inserts (STP004). Step 1: Prior to cell seeding, add 9 ml of media to the insert in a 6-well plate, so the two reservoirs of media are initially separate. 0.5 million cells are seeded into the media on top of the membrane to distribute evenly over the surface of the insert. The cells are then incubated for 15 minutes to allow cells to settle onto the membrane. Step 2: Add 0.5 ml of fresh media down the wall of the insert to ensure the two reservoirs of media are now connected through the opening in the wall of the well insert. Incubate the cultures at 37 °C and 5 % CO2 for 4 days without media changes. Step 3: After 4 days the media is removed, membranes are washed in PBS and trypsin EDTA is added to the membrane. Incubate at 37 °C with agitation on a rotator plate for 7 minutes. Scrape the cells from the membrane using a cell scraper as shown in Step 3. Neutralise the Trypsin EDTA using cell culture media and count cells using a standard haemocytometer and a Trypan Blue exclusion assay. For further passaging and maintenance of the cell population in 3D, repeat Steps 1-3 by seeding 0.5 million cells onto fresh Alvetex Strata membranes as in Step 1. Seed excess cells into additional well inserts to expand the cell population.
The propagation of TERA2.cl.SP12 cells on Alvetex Strata is clearly described in the section on Methodology herein and shown schematically in Figure 2. When grown in 2D culture, TERA2.cl.SP12 cells have a characteristic pluri-potent stem cell morphology comprising a nucleus with prominent nucleoli and a thin rim of cytoplasm (Figure 3A). A large pool of such cells was expanded in conventional tissue culture flasks and then split 50:50 and subsequently passaged in two different ways. Cells were either maintained continually in 2D culture on conventional plasticware or maintained continually in 3D culture on Alvetex Strata. The 3D approach involves growing and maintaining cells on the surface of the porous membrane (Figure 3B). Cells can be visualized using standard histological techniques or other methods (see protocols). Cells in im-mediate contact with Alvetex Strata retained a 3D structure and were unable to spread out due to the rough surface topography presented by the porous material. As more cells settled down on the material and cell proliferation progressed, cells be on the surface creating dense multiple layers up to 40-50μm thick. TERA2.cl.SP12 cells were maintained on the surface of Strata for 3-4 days before they were passaged onto a fresh Strata membrane. Whilst a small number of cells entered the material during this time, the vast majority remained on the surface and any cells within the material were left behind after each passage. Cells were readily removed from the surface of Alvetex Strata, counted, and then re-seeded onto a fresh Strata membrane according to the Method herein (Figure 3C). This enabled continual passaging of cells on the surface of Alvetex Strata where cells were unable to spread out and adopt abnormal morphologies as in conventional 2D cell culture.
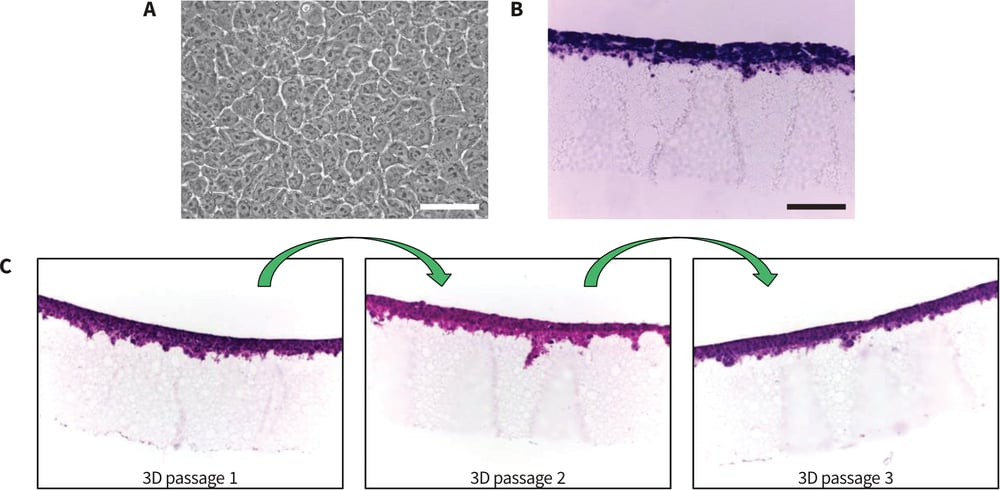
Figure 3: Morphology of human pluripotent stem cells cultured on conventional polystyrene plasticware and Alvetex Strata. A: Phase contrast image of TERA2.cl.SP12 cells grown as flat monolayer cultures on conventional 2D plastic.
B: Histological image showing H&E stained TERA2.cl.SP12 cells that were cultured for 4 days on the surface of Alvetex Strata. Cells form dense multiple layers approximately 40-50μm thick. Individual cells cultured on Alvetex Strata do not spread out and flatten (B) unlike their counterparts grown on flat smooth polystyrene substrates (A). Every 4 days, cells can be passaged from one Alvetex Strata to the next (C). This maintains their 3D shape and prevents them spreading out and adopting thin flattened morphologies as in conventional 2D culture. Scale bars: (A) 25μm; (B) 100μm.
It is well known that when cells are propagated in convention 2D culture, their rate of proliferation increases. In a simple experiment, it was observed that TERA2.cl.SP12 cells maintained their standard high rate of cell proliferation when cultured continuously in conventional 2D plasticware (Figure 3). In contrast, however, when cells were passaged in 3D their proliferation rate was significantly lower by 50-70 % compared to their 2D passaged counterparts (Figure 4). These data indicate that TERA2.cl.SP12 cells passaged in 3D culture on Alvetex Strata develop a constant and lower cell proliferation rate over time.
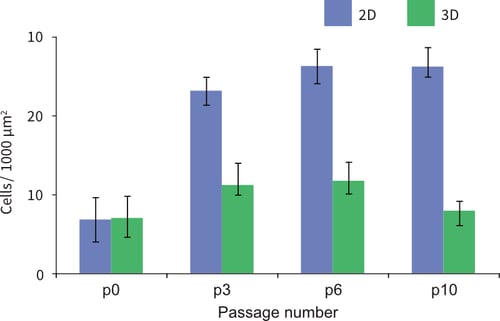
Figure 4: Passaging TERA2.cl.SP12 cells in 3D results in a lower cell proliferation rate over time. After each passage, 200k cells from either 2D culture in conventional plastic-ware or 3D culture on Alvetex Strata were seeded back onto standard 2D tissue culture plastic. These cells were subsequently maintained for 4 days prior to measuring the cell density per unit area. The data show that as passage number increases the density of the cells grown in 3D remains low and relatively constant whereas numbers of cells propagated in 2D increase to higher levels. Given that identical numbers of cells were seeded initially, these data suggest that cells propagated contin-uously in 3D maintain a lower proliferation rate. p0 represents the starting population of cells prior to 4 days growth. Data represent mean, ±SEM, n=10.
TERA2.cl.SP12 cells maintained in 3D culture for prolonged periods began to show evidence of structural changes as the passage number increased. Cells propagated for ×10 passages either in conventional 2D plasticware or in 3D on Alvetex Strata were subsequently seeded back onto 2D culture for 4 days and their structural phenotype compared (Figure 5). Phase microscopy of cultures seeded at lower densities to visualize individual cells, showed that 2D passaged cells spread out as normal whereas those cultured on Alvetex Strata did not spread and possessed a more circular phenotype (Figure 5A). This observation was confirmed by confocal analysis examining the heights of the cells above the 2D culture substrate. On average the height of TERA2.cl.SP12 cells passaged in 3D on Alvetex Strata was approximately 50 % greater than cells passaged in conventional 2D culture (Figure 5B). Collectively, these data indicate that TERA2.cl.SP12 cells have adapted to their surroundings during prolonged exposure to their different microenvironments.
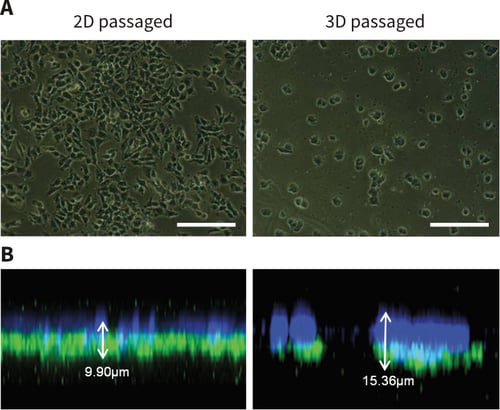
Figure 5: Long-term propagation of pluripotent stem cells in 3D culture results in structural changes and adaptation to the growth substrate. Phase contrast (A) and confocal (B) imaging demonstrate the morphological differences between cells propagated in either 2D or 3D culture for 10 passages. A clear difference in cell morphology develops as passage number increases. Phase micrographs (A) show that 3D passaged cells appear rounder and are more likely to grow in colonies as cell numbers increase than the 2D cells which form a monolayer of more irregular shaped flattened cells. Confocal imaging (B) of cells stained with DAPI (blue), phalloidin (green) and α-tubulin (red) produced Z-stacks of the monolayer show a clear difference in height between cells propagated either in 2D or 3D. Scale bars: 100μm.
Microscopic analysis showed that TERA2.cl.SP12 cells changed their morphology in response to growth and continual propagation in 3D culture using Alvetex Strata. Subsequent experiments were performed to investigate whether other aspects of the cell phenotype were altered. First, the expression of the stem cell marker, SSEA-3, was examined using flow cytometry (Figure 6A). TERA2.cl.SP12 cells maintained in 2D culture show strong expression of SSEA-3 with approximately 50-70 % of the cell population expressing high levels of the stem cell marker. Maintenance of the cells in 3D culture resulted in significantly enhanced levels of SSEA-3 expression in up to approximately 70-90 % of the cell population after ×3 passages. To further confirm the expression of stem cell markers, immunocytochemistry was performed to localize Oct 4 expression in 2D and 3D cultures of TERA2.cl.SP12 cells (Figure 6B). Cells cultured in 3D on the surface of Alvetex Strata stained intensely indicating high levels of Oct 4 expression. Overall, these data suggest that maintaining TERA2.cl.SP12 stem cells in 3D on Alvetex Strata promotes and maintains their stem cell phenotype.
A series of experiments were then performed to assess the developmental potential of the pluripotent stem cells after their propagation either in 2D or 3D culture. Three different approaches were used, all involving the growth and differentiation of TERA2.cl.SP12 stem cells in 3D but in different ways as follows:
- Differentiation in response to retinoid in 3D using Alvetex Scaffold;
- Formation of 3D cell aggregates in suspension culture;
- Transplantation of cells into mice and formation of teratomas.
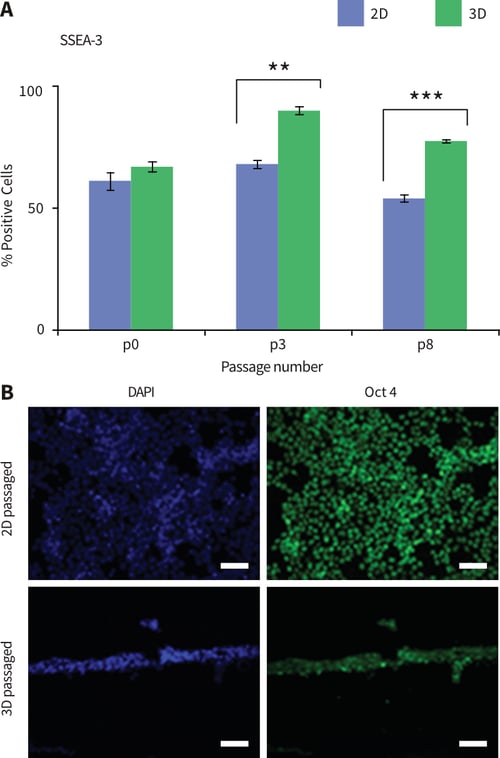
Figure 6: Passaging pluripotent stem cells in 3D results in the enhanced expression of stem cell markers. A: TERA2.cl. SP12 cells propagated and maintained in 2D or 3D culture were analysed by flow cytometry for the expression of the stem cell marker SSEA-3 at passages P0, P3 and P8. Cells expressed higher levels of SSEA-3 when maintained in 3D culture compared to conventional 2D culture. Data represent mean, ±SEM, n=3, **p=0.01, ***p=0.001. B: Immunocytochemical staining showed that TERA2.cl.SP12 cells expressed high levels of the stem cell marker Oct 4 when passaged and maintained in either 2D or 3D culture. Scale bars: 100μm.
Growth and Differentiation in Alvetex Scaffold
After ×10 passages growth in 2D on conventional plastic-ware or 3D on Alvetex Strata, TERA2.cl.SP12 cells were harvested and subsequently seeded onto Alvetex Scaffold and maintained for 14 days in the presence of REPROCELL*’s synthetic retinoid ec23. It has previously been shown that ec23 is a potent inducer of neurogenesis in TERA2.cl.SP12 cells (Christie et al. 2008). Two important observations were made from the current experiment: (1.) Cells that had previously been grown in 3D culture, appeared to penetrate and populate the Alvetex Scaffold more readily compared to their 2D cultured counterparts. Both 2D and 3D passaged cells grew well in 3D within Alvetex Scaffold and penetrated the material throughout. Images from histological preparations clearly show however, that scaffolds that received 3D passaged cells subsequently formed more established 3D cultures (Figure 7); (2.) The developmental potential of 3D cultured TERA2.cl.SP12 cells appeared to be enhanced. Immuno-cytochemical staining for the neural marker, TUJ-1, showed significantly more intense staining and evidence of enhanced neurogenesis in cells previously passaged in 3D compared to 2D (Figure 7). Together these data indicate that the propagation of TERA2.cl.SP12 cells on Alvetex Strata had a beneficial effect on their subsequent ability to adapt to 3D culture and also enhanced their capacity to differentiate and form neural tissues.
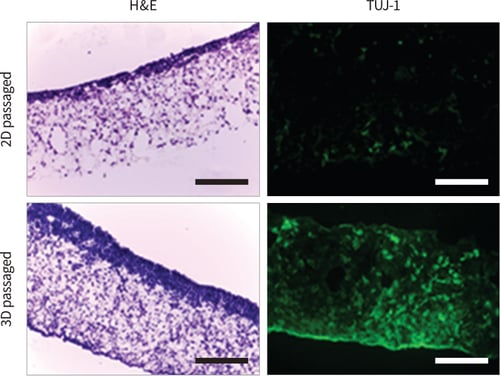
Figure 7: Passaging pluripotent stem cells in 3D subsequently results in their enhanced growth and differentiation in Alvetex Scaffold. TERA2. cl.SP12 cells were propagated for 10 passages in 2D or 3D culture and subsequently seeded onto Alvetex Scaffold. Cells were maintained for 14 days in 3D culture on the scaffolds during which they were exposed to 1μm ec23, a potent synthetic retinoid, to induce neural differentiation. 2D and 3D passaged cells grown and differentiated on Alvetex Scaffold were fixed and processed for histological analysis and immunocytochemical detection of the neural marker, TUJ-1. H&E staining showed significantly enhanced growth of 3D passaged cells compared to their 2D passaged counterparts. In addition, many more TUJ-1positive cells were identified in cultures of 3D passaged cells, indicating enhanced neural differentiation. Scale bars: 100μm.
Formation of 3D cell aggregates
Pluripotent stem cells are readily amenable for growth as cell aggregates in suspension. Such cellular aggregates are known as embryoid bodies and contain differentiated cells representative of primitive embryonic tissues. These structures are 3D in nature and provide a useful assessment of the developmental potential of stem cells. Experiments were carried out with TERA2.cl.SP12 cells to assess their ability to form cell aggregates after different growth periods in either 2D or 3D culture. First, the size of the aggregates was assessed in relation to passage number. As the number of passages increased, the size of the aggregates became significantly greater when cells had previously been passaged in 3D using Alvetex Strata (Figure 8A). By passage 10, aggregates derived from 3D cultured cells were approximately 25 % larger than those passaged in conventional 2D plasticware. Second, the differentiation of 2D and 3D passaged TERA2.cl.SP12 cells was assessed when grown as aggregates in the presence of retinoid. Aggregates derived from cells previously cultured on Alvetex Strata showed greater structural diversity and stained positive for markers of neural and epithelial cell types (Figure 8B). In contrast, aggregates from 2D passaged cells were less heterogeneous and primarily showed staining for neural tissues. Again, these data indicate that the passaging of TERA2.cl.SP12 stem cells on Alvetex Strata had a positive effect on their subsequent ability to adapt to 3D culture and also enhanced their capacity to differentiate.
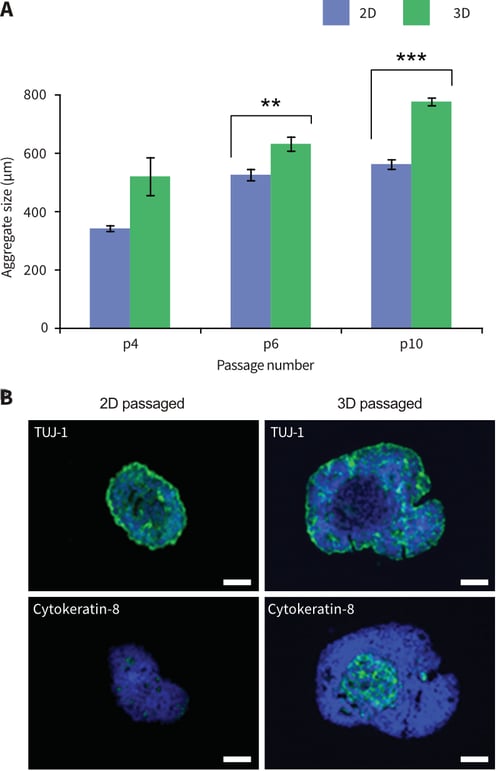
Figure 8: Passaging pluripotent stem cells in 3D results in their enhanced growth and differentiation when sub-sequently cultured as 3D suspended cell aggregates. TERA2.cl.SP12 cells were propagated for 4-10 passages in 2D or 3D culture and then cultured in suspension to form aggregates. Cells maintained in 3D for 6-10 passages formed significantly larger diameter aggregates com-pared to cells continually propagated in 2D culture (A). Data represent mean, ±SEM, n=10, **p=0.01, ***p=0.001. Aggregates were immuno-stained for the neural maker TUJ-1, and the epithelial marker cytokeratin-8 (B). Cells passaged in 3D resulted in aggregates with greater cellular heterogeneity including intense TUJ-1staining and areas with lower cell density expressing cytokeratin-8. Cells propagated in 2D culture formed smaller aggregates that were primarily TUJ-1positive. Scale bars: 100μm.
Formation of teratomas
The gold standard test to determine the developmental potential of pluripotent stem cells involves their transplantation into an appropriate host and the formation of a teratoma (Przyborski, 2005). Human pluripotent stem cells are most often grown as xenograft tumours in immune deficient mice. The resulting tumours are examined using histological methods to show the structural diversity of tissues differentiated from the engrafted stem cell population. In this study, pluripotent TERA2.cl.SP12 cells passaged in either 2D or 3D culture were prepared for transplantation into mice using standard procedures. An initial assessment of the tumours produced focused on their size and mass. Simply weighing the dissected tumour tissue showed significant differences between the xenografts derived from TERA2. cl.SP12 cells cultured in different ways (Figure 9A). After ×10 passages, tumours derived from cells cultured on Alvetex Strata were on average almost 50 % larger than those derived from 2D cultured cells. Histological analysis of the samples showed that each tumour type was solid and possessed structural diversity. Example images of such tumours are shown in Figure 9B, which also demonstrates the difference in tumour size.
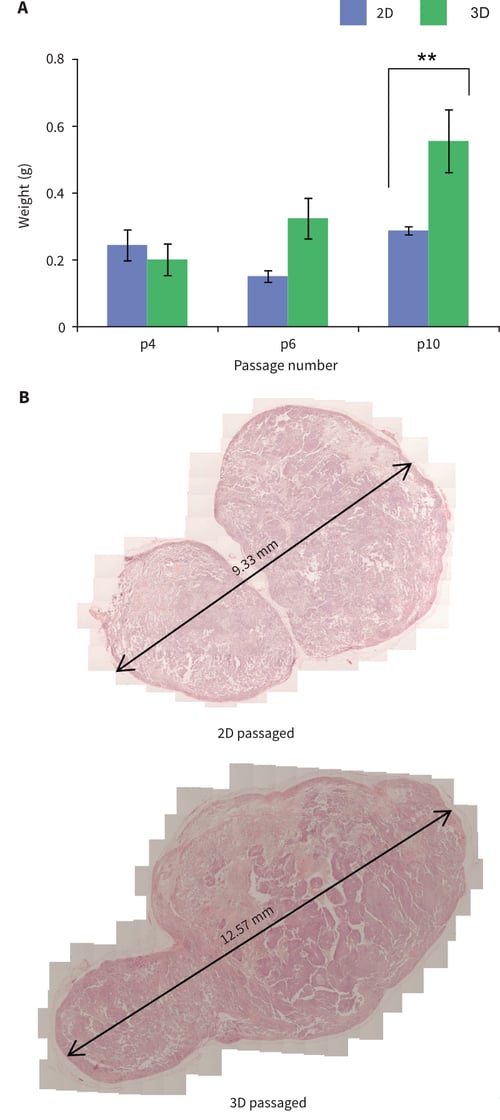
Figure 9: Passaging pluripotent stem cells in 3D results in their enhanced tumour growth when subsequently grown as xenografts in immune deficient animals. TERA2.cl.SP12 cells were propagated for 4-10 passages in 2D or 3D culture and then transplanted into adult Nude mice. (A) Cells maintained in 3D for 10 passages formed significantly larger tumours compared to cells continually propagated in 2D culture. Data represent mean, ±SEM, n=3, **p=0.01. (B) Example histological analysis of teratomas formed from cells propagated continually for 10 passages either in 2D or 3D culture.
Histological imaging alone was not sufficient to determine the diversity of the different tissues within the xenograft tumours produced. Immunocytochemical staining was used to examine the expression of the stem cell marker Oct 4 and markers indicative of ectoderm (neural β-III-tubulin and cytokeratin-8), mesoderm (smooth muscle actin), and Oct 4 staining were observed in xenografts derived from 2D cultured TERA2.cl.SP12 stem cells, whilst only low levels of Oct 4 expression were observed in tumours derived from 3D passaged cells. This may suggest that fewer stem cells exist and differentiation is more complete in xenografts derived from 3D passaged cells. TERA2.cl.SP12 cells are known for their ability to form ectodermal derivatives including neural and epithelial tissues (Przyborski et al. 2004). Both forms of tumour expressed markers for neural and epithelial cells although the amount of expression for both tissue types appeared greater in xenografts derived from 3D passaged cells. The presence of substantial numbers of endodermal and mesodermal cells in tumours derived from TERA2.cl.SP12 cells previously cultured in conventional 2D plasticware has not been reported previously. Staining for smooth muscle actin and α-feto-protein was significantly greater in tumours produced from 3D passaged cells compared to those derived from their 2D cultured counterparts, where only small areas of low-level staining was observed (Figure 10). Collectively, these data are consistent with earlier findings report above, suggesting that the passaging of TERA2.cl.SP12 stem cells on Alvetex Strata had a favorable influence over their ability to adapt to 3D growth after transplantation and significantly enhanced their developmental potential.
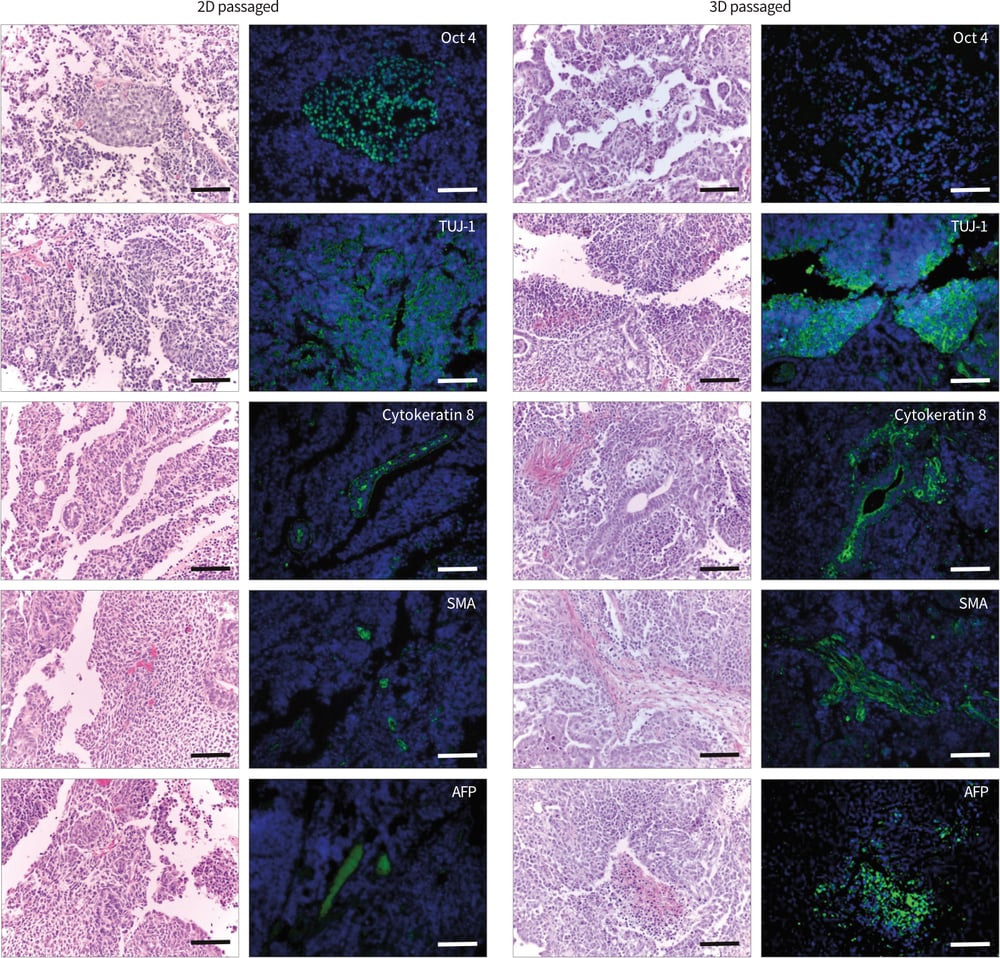
Figure 10: Passaging pluripotent stem cells in 3D results in their enhanced differentiation when subsequently grown as xenografts in immune deficient animals. Data show TERA2.cl.SP12 cells propagated for 10 passages in 2D or 3D culture and then transplanted into adult Nude mice. Tumour tissues were immunostained for Oct-4, TUJ-1, cytokeratin-8, smooth muscle actin (SMA) and α-feto-protein (AFP). Cells passaged in 2D formed teratomas that were mainly TUJ-1 positive with some areas of Oct-4 but limited expression of other markers. In contrast, cells passaged in 3D formed tissues that stained strongly for TUJ-1, cytokeratin-8, SMA and AFP but with only weak Oct-4 localized to a few cells. Scale bars = 100μm.
The data presented in this application note about the use of Alvetex Strata for propagation of cells in 3D has been based on the culture of the human embryonal carcinoma stem cell line, TERA2.cl.SP12. Additional experiments were also performed to demonstrate the principle of passaging human embryonic stem cells in 3D using Alvetex Strata (Figure 11). Although extensive analysis of these cells has not been completed at this stage, it is clear that propagation of embryonic stem cells is also feasible using similar approaches. Histological analysis showed RC-10 human embryonic stem cells massing on the surface of the Alvetex Strata membrane (Figure 11A). Immunocytochemical staining of these cells showed that they were Oct4 positive indicating that they maintain their stem cell phenotype (Figure 11B).
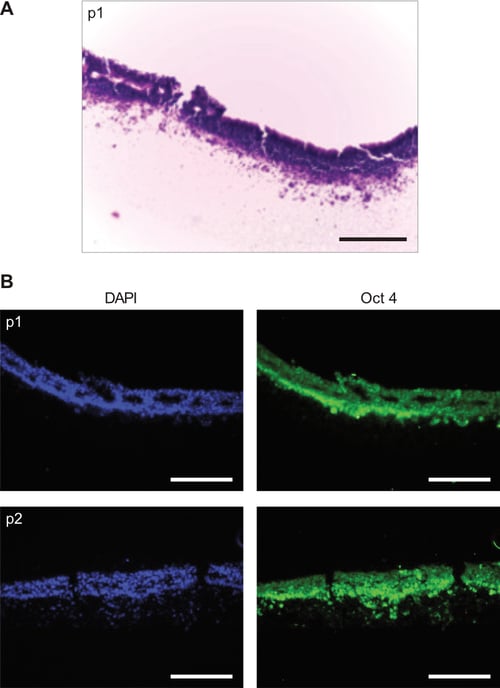
Figure 11: Propagation of human embryonic stem cells (clone RC-10) on Alvetex Strata. Proof of concept experiments were performed to demonstrate that human embryonic stem cells could also be grown and maintained in 3D through serial passaging on Alvetex Strata mem-branes in a similar fashion to human EC stem cells. A: H&E stained section of RC-10 cells during first passage on Alvetex Strata. B: Immunostaining shows high levels of Oct 4 expression in RC-10 cells passaged every 3-4 days on the surface of Alvetex Strata. Scale bars = 100μm.
Conclusions
Alvetex Strata offers a new approach to cultivating cells continuously in 3D to achieve notable improvements in cell growth and function. Strata can be used to routinely maintain proliferative cells with a 3D structural phenotype in preparation for their use in down-stream assays that involve 3D cell culture or cell transplantation. Specifically, we have demonstrated an application to show how stem cells can be propagated continuously in 3D on the surface of Alvetex Strata. The consequences of such cell growth result in adaptation of cells to their surroundings and the acquisition of a more appropriate 3D architecture. This in turn appears to have a positive influence on the function and developmental potential of stem cells.
Methodology
Culture of embryonal carcinoma stem cells in 2D using conventional plasticware
- Initial stocks of TERA2.cl.SP12 cells were grown and cell numbers expanded in conventional 2D culture flasks according to previous methods (Przyborski, 2001).
- Cells were maintained in T75 flasks (BD Bioscience) in high glucose Dulbecco’s Modified Eagles Medium (DMEM, Invitrogen) supplemented with 10 % fetal calf serum (Invitrogen), 2 mM L-glutamine and penicillin/ streptomycin (Lonza). All TERA2.cl.SP12 cultures were maintained at 37 °C and 5 % CO2.
- Rolling acid washed glass beads over confluent cell cultures every 2-3 days and splitting 1:3 was performed to passaged the cells. Cell suspensions from T75 flasks were prepared using 2 ml 0.2 % Trysin EDTA (Lonza) and cell number determined by counting on a haemocytometer.
Propagation of embryonal carcinoma stem cells in 3D using Alvetex Strata
- Membranes of Alvetex Strata mounted in 6-well inserts were ethanol-wetted in 70 % ethanol and washed twice in PBS containing calcium and magnesium. The final PBS wash was removed and 9 ml of media was added to each well of a standard 6-well multi-welled plate containing a washed Alvetex Strata 6-well insert.
- Cells were trypsinised from T75 flasks and counted using a haemocytometer and Trypan Blue exclusion assay. The cells were centrifuged and re-suspended in media to allow for 0.5 × 106 cells in 500 μl for each disc of Alvetex Strata.
- The 500 μl of cell suspension was added to the well containing Strata inserts with the 9ml of media using a dispersed seeding technique to distribute the cells over the surface of the Alvetex Strata membrane (see Figure 2, Step 1).
- The cultures were then incubated for 15 minutes at 37 °C and 5 % CO2 to allow for cells to settle onto the membranes. After incubation, 500 μl of fresh media was added down the side of the insert to allow for the two reservoirs of media to become connected (see Figure 2, Step 2).
- Cultures were then incubated at 37 °C and 5 % CO2 for 24 hours. The media was then removed and carefully replaced with 10 ml of fresh media. Cultures were incubated for a further 3 days with no media changes to avoid disturbing the dense cell layer.
- After 4 days of growing TERA2.cl.SP12 cells on the surface of Alvetex Strata, the well insert was carefully disassembled by un-clipping the base to release the membrane. Handle the membrane carefully using a blunt pair of tweezers.
- The membranes were washed in PBS without calcium and magnesium and placed in the bottom of a 6-well multi-welled culture plate. To each well containing a membrane, 2 ml of 0.25 % trypsin EDTA was added and the plate was incubated at 37 °C on an orbital shaker at 100 RPM for 7 minutes.
- After incubation the cells were scraped from the membranes using a flexible cell scraper (SARSTEDT Ltd 18.1832 – 16 cm length & 1.3 cm blade) (see Figure 2, Step 3).
- The trypsin EDTA was then neutralised using 3 ml of cell culture media. Cell suspensions from individual discs were combined in a Falcon tube, centrifuged at 1000 RPM for 3 minutes and the cell pellet was re-suspended in 5 ml of cell culture media. Cells were counted using a Trypan Blue exclusion assay and a haemocytometer and then re-suspended to a dilution of 0.5 × 106 cells per 500 μl.
- The cells were then re-seeded at a concentration of 0.5 × 106 cells per 500 μl onto freshly prepared new Alvetex Strata membranes to complete the passaging process (see Figure 2, Steps 3 to 1).
Note that it is necessary to determine cell number after every passage as cell proliferation slows after prolonged culture in 3D.
Culture of embryonic stem cells in 2D using conventional plastic-ware
- The human embryonic stem cell line RC-10 (Roslin Cellab) was grown as a feeder-free culture using CELLstart coating (Invitrogen) and the StemPro® hESC serum free media kit (Invitrogen).
- Cells were cultured in 6-well plates coated with CELLstart. The coating was diluted to 1:50 in PBS containing calcium and magnesium prior to the addition of 750 μl of the solution to each well of the plate and incubated for 1 hour at 37 °C.
- The StemPro kit is a serum and feeder-free media based on DMEM/F12 with Glutamax supplemented with 25 % bovine serum albumin (BSA), StemPro hESC growth supplement (50×) and 10 μg/ml basic fibroblast growth factor (bFGF).
- Complete media consists of 454 ml DMEM/F12, 10 ml of 50× StemPro supplement, 36 ml of 25 % BSA to a give a final concentration of 8 % and finally 400 μl of the 10 μg/ml stock solution of bFGF for a final concentration of 8 ng/ml.
- RC-10 cells were thawed at 37 °C and transferred to a centrifuge tube containing 4.5 ml of warmed complete StemPro media. The cells were pelleted by centrifuging at 1300 RPM for 3 minutes. The supernatant was removed and 5 ml of complete media was added and the cell pellet was carefully broken up by pipetting.
- CELLStart solution was removed from one well of a 6-well plate and the 5 ml of cell suspension was added. Cells were incubated at 37 °C and 5 % CO2 for 24 hours to allow cells to adhere before 100 % of the media was changed to remove cell debris.
- Cells were passaged using a manual dissociation technique. Media was removed from the cell layer and 3 ml of fresh StemPro media was added. RC-10 cells were scraped from the bottom of the well using a 1000 μl blue pipette tip, and the resulting cell suspension was split between 8 CELLStart coated wells. Media was change every 48 hours until cultures reached 70-80 % confluent. Cells were split 1:8 once a week.
Propagation of embryonic stem cells in 3D using Alvetex Strata
- Alvetex Strata membranes mounted in 6-well inserts were treated with 70 % ethanol and washed twice in 5 ml of sterile deionised water, before being coated with CELLStart.
- As previously described above for 2D cultures, the CELLStart coating was diluted to 1:50 in PBS containing calcium and magnesium prior to the addition of 750 μl of the solution to each Strata membrane. Alvetex Strata inserts were placed on the bottom of a Petri dish for the coating procedure. Coated membranes were incubated for 1 hour at 37 °C.
- RC-10 embryonic stem cells were removed from the conventional 2D 6-well plate that they had been cultured in using Accutase solution (Sigma Aldrich, UK). The cells were dissociated to form a single cell suspension and cell number was determined using a Nucleo-counter NC-3000 (ChemoMetec A/S).
- The inoculation volume was calculated based on the viable cell count only and membranes were seeded with 1 × 106 cells each. Membranes containing cells were cultured in 10 ml StemPro media for between 3.5 days to 7 days between each passage. Media was changed every 48 hours.
- For passaging RC-10 cells on Alvetex Strata, inserts containing membranes were gently washed twice in PBS with calcium and magnesium and then placed into a Petri dish containing 20 ml of warmed Accutase. Inserts were allowed to sit undisturbed at room temperature for 15 minutes. During this time, 5 ml of fresh StemPro medium was placed into each well of a standard 6-well multiwelled culture plate.
- After incubation the Strata inserts were removed from the Accutase solution and placed into the wells containing the StemPro medium. Using a 1000 μl pipette and tilting each individual insert, the cells were removed from the membrane by repeatedly pipetting the media onto the membrane to wash cells into the well. Cells from multiple membranes were collected into centrifuge tubes and centrifuged at 1300 RPM for 3 minutes. Cells were again counted using NC-3000 machine.
- The process was repeated to enable continual propagation of human RC-10 embryonic stem cells on Alvetex Strata by seeding 1 × 106 viable cells onto freshly prepared new membranes following the methods as previously described.
Characterization of stem cells and their developmental potential
Subsequent to the continual propagation of pluripotent stem cells on conventional 2D plasticware or on Alvetex Strata, the phenotype of the cells was assessed using an array of standard methods. These are outlined in brief as follows:
- Monitoring cell proliferation rate: After continual passage on either 2D convention plasticware or on Alvetex Strata membranes, equal numbers of cells were seeded on conventional 2D multi-welled plates as maintained as monolayers and for 4 days. Cells were subsequently fixed in 4 % PFA and stained with Hoescht 33342 to highlight nuclei. The number of nuclei was counted in five randomly selected 100 μm squares in 5 separate images for every passage number and culture condition. Cell density was calculated as the number of cells per 100 μm2.
- Flow cytometry: The expression of the stem cell marker SSEA-3 was assessed on TERA2.cl.SP12 cells using flow cytometry in a similar manner as previously described (Przyborski, 2001).
- Differentiation in 3D culture using Alvetex Scaffold: After 10 passages on either 2D conventional plasticware or on Alvetex Strata membranes, TERA2.cl.SP12 cells were transferred to Alvetex Scaffolds to assess their ability to differentiate in 3D. Cells were seeded onto the scaffolds using the optimised protocols (see https://www.reprocell.com/resources/protocols) and cultured for 4 days. Cultures were then maintained for a further 14 days in the presence of the synthetic retinoid, ec23 (1 μM), to induce cell differentiation and the formation of neurons (Christie et al. 2008). Experiments were performed in 6-well inserts (AVP-004) housed in the deep well Petri dish (AVP-015). After 14 days induced differentiation, 3D cultures were fixed in 4 % PFA and processed for tissue sectioning. Immunocytochemistry was performed to examine the expression of the neural marker TUJ-1 (Cambridge bioscience PRB-435P-100 at 1:600) following recommended methods (see protocols.
- Differentiation in 3D culture using cell aggregate method: Aggregates were formed from TERA2.cl.SP12 cells using a suspension culture method. Cells were trypsinised from their cultured substrates (2D conventional plasticware or Alvetex Strata) and counted using the Trypan Blue exclusion assay. Cell suspensions were centrifuged at 1000 rpm for 3 minutes and the cell pellet was re-suspended at a concentration of 1 × 107 cells per ml. 100 μl of this cell suspension was added to 10 ml of complete DMEM in a non-coated bacteriological Petri dish. After 24 hours, synthetic retinoid ec23 was added to the Petri dishes to a final concentration of 1 μM. Cells were allowed to form aggregates and media was changed every 2-3 days by transferring aggregates to a 50 ml centrifuge tube and allowing them to settle to the bottom of the tube. After 10 minutes the spent media was removed and aggregates were carefully resuspended in 10 ml of fresh media containing 1 μM ec23. Aggregates were cultured for up to 21 days, phase images were captured, and measurements recorded. Cell aggre-gates were also fixed and prepared for immunocytochemistry using the antibodies TUJ-1 (Cambridge bioscience PRB-435P-100 at 1:600) and cytokeratin-8 (Sigma C5301 at 1:200).
- Differentiation in 3D using cell transplantation and teratoma formation: All procedures involving mice were conducted in accordance with guidelines and permission granted by the Institution and the Home Office, UK. Cells were trypsinised from either 2D conventional plasticware or Alvetex Strata. Viable cells were counted using the Trypan Blue assay and 0.5 × 106 cells were re-suspended in 100 μl of culture media. Each cell suspension was combined with 100 μl of BD Matrigel (BD Bioscience) to increase the success rate of tumour formation. The resulting 200 μl of cell suspension was loaded into 1ml syringes with 21G needles. Cells were injected subcutaneously into adult male Nude (nu/nu) mice. Mice were examined regularly and when teratoma formation was visible the tumour was measured at least once a week until they reached approximately 1 cm3. At this point mice were sacrificed and teratoma tissue was surgically removed and weighed. This was usually between 6-8 weeks after injection. Tumours samples were prepared for histological analysis following standard methods (see Cooke et al. 2006). To assess the presence of tissues representative of the three germ layers, immunocytochemistry was performed using antibodies reactive to markers of ectoderm, mesoderm and endoderm: namely, β-III-tubulin (TUJ-1, 1:600, Cambridge Bioscience PRB-435P-100); smooth muscle actin (SMA, 1:100, Abcam ab5694); α-feto-protein (AFP, 1:500, Sigma Aldrich A8452), respectively. The stem cell marker oct4 (Abcam, ab19857, 1:250) and epithelial marker cytokeratin-8 (Sigma, C5301, 1:200) were also examined.
References
- Bissell MJ, Rizki A, Mian IS (2003). Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol 15:753-62.
- Christie VB, Barnard JH, Batsanov AS, Bridgens CE, Cartmell EB, Collings JC, Maltman DJ, Redfern CP, Marder TB, Przyborski S, Whiting A (2008). Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org Biomol Chem 6:3497-507.
- Cukierman E, Pankov R, Stevens DR, Yamada KM (2001). Taking cell-matrix adhesions to the third dimension. Science 294:1708-12.
- Cukierman E, Pankov R, Yamada KM (2002). Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 14:633-9.
- Nelson cm, Bissell MJ (2006). Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287-309.
- Przyborski SA (2005). Differentiation of human embryonic stem cells after transplantation in immunedeficient mice. Stem Cells 23:1242-50.
- Przyborski SA, Christie VB, Hayman MW, Stewart R, Horrocks GM (2004). Human embryonal carcinoma stem cells: models of embryonic development in humans. Stem Cells Dev 13:400-8.
- Przyborski SA (2001). Isolation of human embryonal carcinoma stem cells by immunomagnetic sorting. Stem Cells 19:500-4.
- Schmeichel KL, Bissell MJ. (2003). Modeling tissuespecific signaling and organ function in three dimensions. J Cell Sci 116:2377-88.
- Schutte M, Fox B, Baradez MO, Devonshire A, Minguez J, Bokhari M, Przyborski S, Marshall D (2011). Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three-dimensional culture on a polystyrene scaffold designed for routine use. Assay Drug Dev Technol 9:475-86.