Alvetex Scaffold Application Note 06
Application of Alvetex Scaffold for 3D Culture of Osteocytes to Investigate Regulation of Bone in Normal and Simulated Microgravity Environments
Download this application note as a PDF (5.1 MB)
In this application note we describe work carried out by Jordan Spatz, a PhD student at Harvard MIT Division of Health Sciences & Technology (HST) working with Professor Paola Divieti-Pajevic. Some of this data has been previously presented as a scientific poster at the 2012 Annual Society for Bone and Mineral Research meeting. We would like to thank Jordan and Paola for allowing us to show these data and images of their work on the 3D culture of Osteocytes.
Introduction
An improved understanding of bone density under conditions of microgravity may increase our understanding of how the structure and physiology of the skeleton changes during the absence of prolonged weight bearing and inactivity. This application note presents preliminary data obtained from 3D cultures of a novel osteocyte cell line in Alvetex Scaffold, under simulated microgravity conditions. The work forms part of a larger programme that will see similar experiments conducted on the International Space Station (ISS), funded by the National Institutes of Health BioMed-ISS initiative in 2014 (NIH-AR059655).
Osteocytes are a major cell type found in adult bone. Once thought to relatively inert and they are now known to act as mechanosensory cells that exert bone-remodelling control through interaction with bone building cells, osteoblasts, and bone resorbing cells, osteoclasts. To date our understanding of osteocytes has been hampered by poor availability of model cell lines. Recently, two osteocyte-like cell lines have been isolated from the bones of genetically engineered mice (SV40 antigen-expressing mouse crossed with a mouse expressing GFP under the control of the Dentin Matrix Protein 1 promoter, a late osteoblast to early osteocyte promoter). Clones were selected for high SOST expression and PTH (parathyroid hormone) responsiveness. Importantly, these cell lines have hallmark expression of an osteocyte phenotype by expressing FGF23 and SOST (Sclerostin). In addition, these cell lines displayed osteocytic morphology (dendritic) and low expression of osteoblast markers.
The osteocyte cell line Ocy454 was cultured in Alvetex Scaffold to investigate the effects of 3D cell culture on osteocyte gene expression, both under standard culture conditions and under conditions simulating a micro-gravity environment.
Results and Discussion
Analysis by phase contrast microscopy showed that the Ocy454 osteocyte cell line exhibited morphology typical of that normally associated with osteocytes when grown in conventional 2D culture dishes (Figure 1).
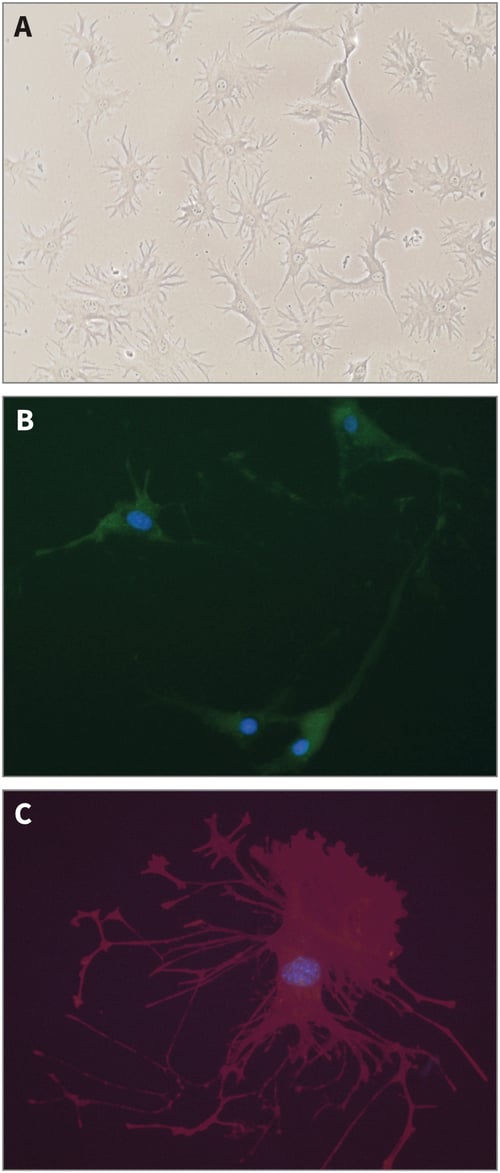
Figure 1: Structure of Ocy454 osteocyte-like cells in conventional 2D culture. (A) Phase micrograph (using ×20 objective lens); (B) GFP fluorescence; (C) Phalloidin staining revealing actin cytoskeleton.
In 3D culture, Ocy454 cells grew throughout the internal structure of Alvetex Scaffold (Figure 2). Sclerostin (the protein product of the SOST gene) has emerged as a powerful inhibitor of bone formation. The protein binds to LRP5 and the related LRP6 and 4 receptors and blocks the canonical Wnt-β-catenin signalling pathway and it is known that Sclerostin expression is inhibited by PTH [1,2]. Immunocytochemical staining for Sclerostin revealed strong expression in a proportion of cells throughout Alvetex Scaffold cultures (Figure 2C and 2D). Subsequent treatment with PTH resulted in suppression of Sclerostin expression as evident by less intense staining (Figure 2D). Accordingly, Ocy454 cells grown in Alvetex Scaffold appear to behave in a manner that is consistent with normal osteocyte activity and function.
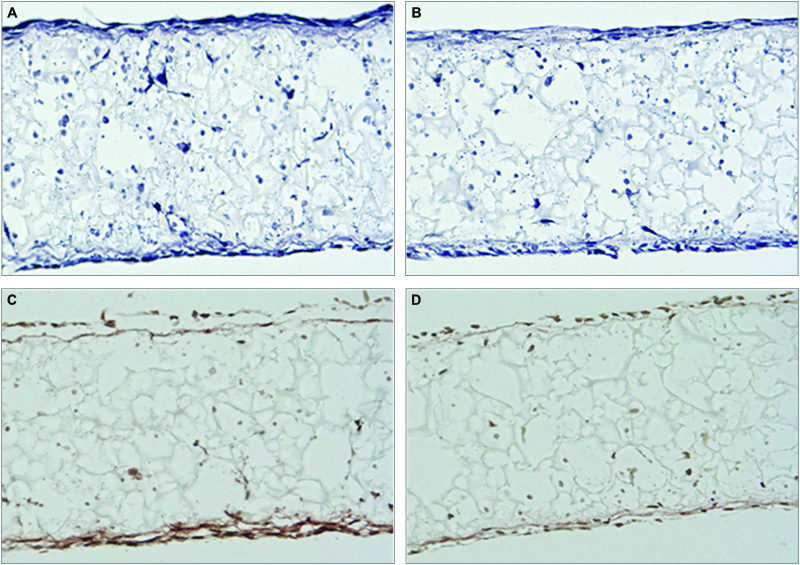
Figure 2: Regulation of Sclerostin expression in Ocy454 cells cultured in Alvetex Scaffold. Ocy454 3D cultures were fixed and analysed by H&E stain (panels A and B); immunocytochemistry, Sclerostin immunostain (panels C and D). Cells in Panel D were treated with PTH for 4hr prior to staining with Sclerostin.
In 3D Alvetex Scaffold cultures of Ocy454, maintained in flight certified bioreactors that will be used to grow cells on ISS there is minimal cells death as shown by Tunel staining in Figure 3 showing the Alvetex Scaffold is an ideal biomaterial for the growth of osteocytes.
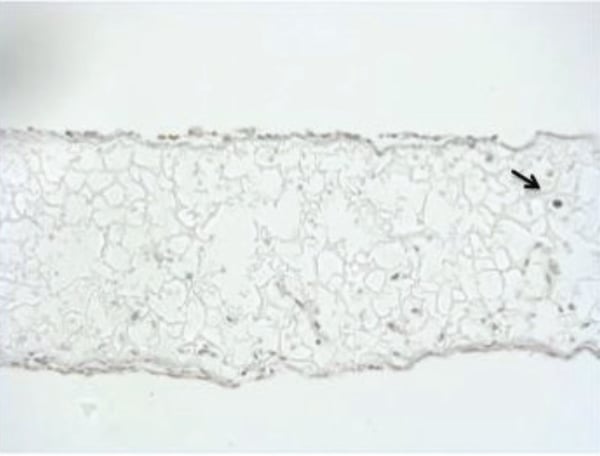
Figure 3: Tunel staining of OCY454 in Alvetex Scaffold. 10 μm sections of paraffin embedded scaffolds were stained by Tunel. Tunel assay (IHC) in cells cultured for 7 days in flight certified bioreactors. The extremely low level of Tunel positive cells (black staining) present under these conditions indicates a very low level of cell death and that cells can be maintained in culture conditions necessary to fly cells to the International Space Station.
Under standard 2D cell culture conditions, the osteocyte cell line Ocy454 expressed significantly higher levels of SOST mRNA than wild type osteoblasts (data not shown). Furthermore, upon treatment with PTH, Ocy454 cells were characterized by increased RANKL and decreased SOST/ Sclerostin expression but showed no change in FGF23 expression.
To further explore the effects of the 3D culture microenvironment on expression and regulation of osteocyte-specific genes, Ocy454 cells were cultured in Alvetex Scaffold for 7 to 14 days. PTH treatment of cells in Alvetex Scaffold resulted in a several fold increase in FGF23 expression. Such a response was not observed in Ocy454 cells grown in conventional 2D culture plates (Figure 4A), suggesting that growth in 3D culture conditions directly enhances the osteocytic phenotype of Ocy454 cells.
The growth of Ocy454 cells was also evaluated in Alvetex Scaffold under simulated microgravity using NASA-Synthecon-Slow Turning Lateral Vessels (STLV) (Figure 4B). Compared with standard control cultures under normal gravity, simulated microgravity gave rise to a four-fold induction of SOST expression. This induction resembles the increase in SOST/Sclerostin observed during in vivo murine models of unloading.
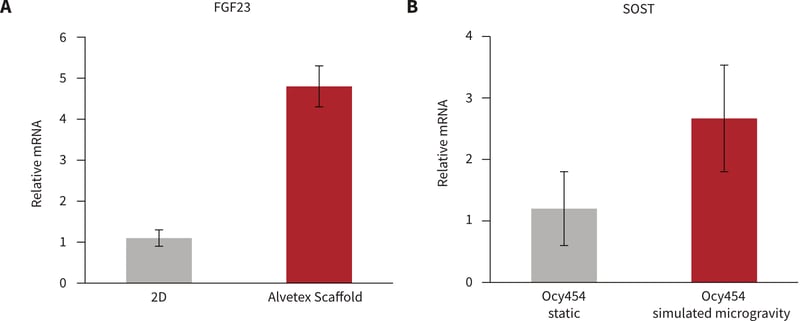
Figure 4: A: FGF23 increases with 100 nM hPTH(1-34) in 3D culture. Ocy454 cells (1.6 million per Alvetex Scaffold disc) grown at 37°C for 2 weeks were treated with 100 nM hPTH(1-34) for 4 hours. B: SOST upregulated by simulated micro-gravity. Ocy454 cells (1.6 million per Alvetex Scaffold disc) grown at 37°C for 11 days placed in static or rotating bioreactors for 96 hours.
Conclusions
The results demonstrate distinct differences in the behavior of osteocytes when placed under alternative growth conditions. Unlike the growth of cells on conventional 2D culture plates, the 3D culture of Ocy454 cells using Alvetex Scaffold technology recreates a more physiologically relevant niche microenvironment that more closely represents the physical surrounding cells experience in the native bone compartment. Moreover, the simulation of microgravity conditions using such models enables researchers to investigate and understand the molecular mechanisms that regulate bone formation and degradation. This will provide valuable insight into the consequences of unloading during time spent in microgravity environments and during extended periods of inactivity or bed-rest. Further experiments using these models and Alvetex Scaffold technology are due to be conducted on board the International Space Station in 2014.
Methods
- Alvetex Scaffold 3D cell culture: Ocy454 cells were seeded onto Alvetex Scaffold at a density of 1.6 × 106 cells per Alvetex 6 well insert (AVP004) following standard manufacturers conditions
- Real-time SYBR® Green qPCR: RNA was isolated and reverse transcribed using commercial kits (Qiagen). PCR detection was performed using a StepOnePlus™ instrument (Applied Biosystems). Results of gene expression analysis experiments are expressed as relative RNA expression and are normalised against beta actin expression. Data are expressed as a mean ± SD. Student unpaired two-tail t-test p<0.05.
- Simulated microgravity: Microgravity was simulated using a rotating vessel bioreactor (NASA/ Synthecon Rotating Wall Vessel).
References
- Bellido T, Saini V, Pajevic PD, Effects of PTH on osteocyte function. Bone 2013, 54(2): 250-7.
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien, CA, Manolagas SC, Jilka RL, Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 2005, 146(11): 4577-83.