Protocol for the Culture of Primary Rat Hepatocytes in Alvetex® Scaffold 96 Well Plate Format
1. Introduction
When using Alvetex Scaffold in well bottom formats (12-well plate, 24-well plate, 96-well plate), hepatocytes are susceptible to dislodgement (for example during media exchange). Therefore when using well bottom formats, it is recommended to use an extracellular matrix coating reagent to facilitate cell attachment. The following protocol is for the culture of cryopreserved rat primary hepatocytes in Alvetex Scaffold 96-well plates.
2. Culture of primary rat hepatocytes in 96-well plate format of Alvetex Scaffold
2.1. Materials
-
Rat cryopreseved primary hepatocytes (Biopredic Int, HEP1 34)
-
Collagen I (BD Biosciences, 354236)
-
Hepatocyte thawing medium: Lebovitz’s L-15 Medium (Lonza, 30-2008)
-
Hepatocyte seeding medium: William’s Medium E (Invitrogen, 22551-022) supplemented with GlutaMAX-1 , 100 U/mL Penicillin/Streptomycin, 10 % (v/v) FBS, 4 μg/mL insulin.
2.2. Pre-coating of Alvetex Scaffold 96-well plate with Collagen I
-
Alvetex Scaffold 96-well plates were prepared for collagen coating by adding 100 μL of 70 % ethanol per well, followed by 1 × wash with 200 μL PBS and 1 × wash with 200 μL 0.02 N acetic acid.
-
To each well 100 μL collagen I was added (BD Biosciences 354236, diluted to 50 μg/mL in 0.02 N acetic acid).
-
Plates were incubated for 1 hour at room temperature.
-
Collagen solution was aspirated and each well was rinsed 3 × with 100 μL hepatocyte seeding medium. The last medium wash was aspirated and the plates placed in a humidified incubator (37 °C / 5 % CO2) until cells were ready for seeding (approximately 30 minutes).
2.3. Seeding of hepatocytes into Alvetex Scaffold 96-well plates
-
Cells were prepared for seeding according to the supplier’s instructions to obtain a cell suspension at a concentration of 2.5 × 105 viable cells/mL.
-
Cells were added directly to the centre of each well, and the total volume per well was immediately made up to 200 μL with hepatocyte seeding medium.
-
Plates were placed in a humidified incubator (37 °C / 5 % CO2).
-
To avoid loss of cells, media was not changed between cell seeding and endpoint analysis.
3. Example data
Primary hepatocytes were seeded into standard 2D and Alvetex Scaffold 96-well plates, at a range of seeding densities, as described above. At 24 hours after seeding, cell viability analysis was performed using a standard MTT assay. Briefly, media was aspirated from each well and immediately replaced with 200 μL 1 mg/mL MTT solution. Plates were incubated at 37 °C / 5 % CO2 for 1 hour. MTT reagent was aspirated and purple formazan product was solubilised by adding 200 μL acidified isopropanol and placing on a shaking platform at 100 rpm for 10 minutes. The product in each well was dispersed by thorough pipetting up and down using a multichannel pipette, followed by transfer of 100 µL from each well into a clean standard 96-well plate for absorbance measurement (see the Alvetex Scaffold 96-well plate instruction booklet for detailed protocols).
MTT curves show similar viability responses for cells cultured in 2D and 3D up to a seeding density of 30,000 cells per well (Figure 1).
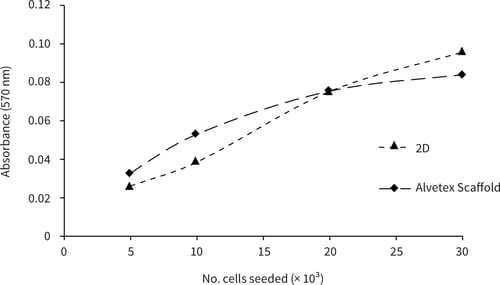
Figure 1. Biochemical analysis of cell viability using a standard MTT assay. Rat hepatocytes (Biopredic Int., HEP134) were seeded at densities of 5, 10, 20 and 30 × 103 cells per well and cultured for 24 hours in either collagen-coated 2D 96-well plates or collagen-coated Alvetex Scaffold 96-well plates (AVP009). Data shown are mean values from triplicate wells.