Guidance Notes and Protocols for the Culture of Primary Rat Hepatocytes in Alvetex® Scaffold in Well Insert and Well Plate Formats
● Download this protocol as a PDF (0.5 MB)
1. Introduction
Liver cells cultured in 3D on Alvetex Scaffold have been demonstrated to be morphologically and functionally superior to counterparts cultured by conventional 2D methods (see reinnervate.com for example protocols, application notes, and peer reviewed literature). Therefore Alvetex Scaffold represents a promising platform for the development of new and improved hepatocyte based in vitro assays.
Cell attachment is a critical factor in successfully establishing a viable hepatocyte culture. Conventional culture of primary hepatocytes on 2D cell culture plasticware requires coating of the culture surface for effective attachment of viable cells. Coating agents typically consist of extracellular matrix (ECM) components such as collagen and fibronectin. The flat, even surface of 2D plasticware promotes strong cell attachment, although this is achieved at the expense of native cellular morphology with cells becoming flattened in monolayer cultures.
In contrast, Alvetex Scaffold provides a surface topography which promotes the adoption of 3D morphology which is typical of hepatocytes in vivo. This topography may compromise the ability of hepatocytes to form strong surface attachments upon initial seeding, and it is therefore recommended that 3D hepatocyte cultures in Alvetex Scaffold are handled with care to minimise or avoid excessive cell loss.
When using Alvetex Scaffold in well bottom formats (12 well plate, 24 well plate, 96 well plate), hepatocytes may be more susceptible to dislodgement (for example during media exchange). Therefore in these formats, it is recommended to consider the use of coating agents, alongside uncoated controls, during growth optimisation (for example coating protocols see Alvetex protocols).
Hepatocytes cultured in well inserts (6-well insert, 12-well insert) can be relatively well protected from disruption during general handling. As such, it is possible to maintain healthy hepatocytes in Alvetex Scaffold well inserts without pre-coating with ECM molecules.
1.1. Culture of primary rat hepatocytes in well insert formats of Alvetex Scaffold
Hepatocyte cultures can be set up in Alvetex Scaffold well inserts (AVP004, AVP005) in one of two ways:
- Contact culture with feeding from beneath
- Submerged culture
Contact feeding eliminates disturbance of cells during media exchange. This method is not recommended for culture periods beyond 1-2 days.
Submerged feeding conditions are established by flooding the outer chamber of the holding well plate followed by gentle introduction of media into the inner chamber of the insert itself. This method does not completely eliminate cell disruption/loss, but cultures may be maintained for longer.
1.2. Culture of primary rat hepatocytes in well bottom (well plate) formats of Alvetex Scaffold
Hepatocyte cultures can be set up in Alvetex Scaffold well plates (AVP002, AVP006) via standard methods, following pre-treatment of the scaffold discs with a suitable coating agent such as Collagen I.
2. Example protocols
Media constituents
Hepatocyte seeding medium
— Williams E Medium supplemented with GlutaMAX-1
— 100 U/mL Penicillin/Streptomycin
— 10 % (v/v) FBS, 4 µg/mL insulin
Hepatocyte culture medium
— Williams E Medium supplemented with GlutaMAX-1
— 100 U/mL Penicillin/Streptomycin
— 4 μg/mL insulin
— 50 μm hydrocortisone 21-hemisuccinate
2.1. Culture of primary rat hepatocytes in Alvetex Scaffold in 12 well insert (AVP005)
-
Alvetex Scaffold 12-well inserts in 12-well plate format were prepared for seeding by dipping in 70 % ethanol followed by media washes (twice with 4 mL per well).
-
75 µL of hepatocyte suspension was added directly to the centre of each Alvetex Scaffold disc (0.2-0.4 × 106 cells per well).
-
The plate was incubated for 30 minutes at 37 °C with 5 % CO2 to allow the cells to settle on the scaffold.
-
Hepatocyte seeding medium was added to each well to achieve either contact or submerged feeding as follows:
-
Contact feeding: 2 mL for 12 well insert in 12 well plate (or 4.0 mL for 12 well insert in 6 well plate).
-
Submerged feeding: 4 mL for 12 well insert in 12 well plate (or 9.0 mL for 12 well insert in 6 well plate).
-
Note: gentle addition of media to minimise disruption to cells.
- Plates were re-incubated for a period of 4 hours, after which medium was exchanged for an equal volume of hepatocyte culture medium.
Note: gentle addition of media to minimise disruption to cells.
2.2. Culture of primary rat hepatocytes in Alvetex Scaffold in 24 well plate (AVP006) format
-
Alvetex Scaffold 24 well plates were wetted by adding 2 mL 70 % ethanol followed by two PBS washes (2 mL each per well).
-
Alvetex Scaffolds were treated with Collagen I (see coating protocols at Alvetex protocols).
-
75 μL of hepatocyte suspension was added directly to the centre of each Alvetex Scaffold disc (0.2-0.4 x 106 cells per well).
-
The plate was incubated for 30 minutes at 37 °C with 5 % CO2 to allow the cells to settle on the scaffold.
-
Hepatocyte seeding medium was added to each well to a total volume of 2 mL.
-
Note: gentle addition of media to minimise disruption to cells.
-
Plates were re-incubated for a period of 4 hours, after which medium was exchanged for 2 mL of hepatocyte culture medium.
Note: gentle addition of media to minimise disruption to cells.
Note: This method can be applied to the use of Alvetex Scaffold in 12 well plate format (AVP002). Adjust cell seeding and media volumes according to the guidelines provided in the Alvetex Scaffold Quick Start Protocol.
3. Example data
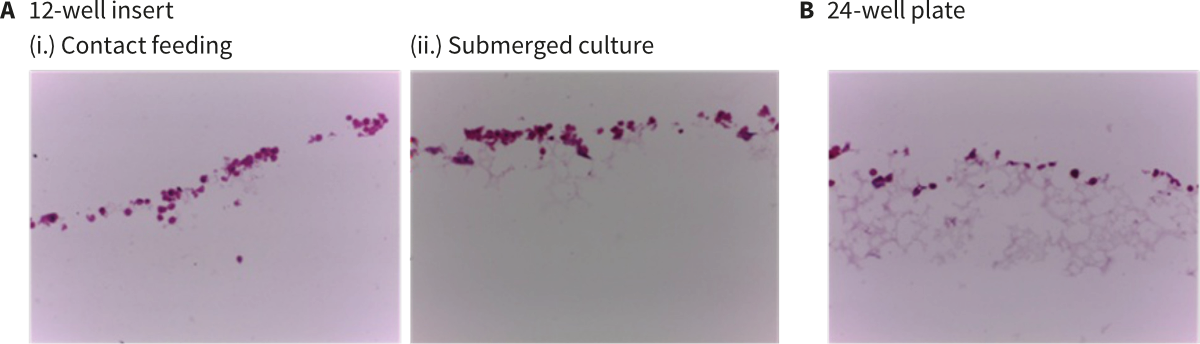
Figure 1. Brightfield micrograph showing the structure of rat hepatocytes (Biopredic Int., HEP134, Male rat cryo-preserved hepatocytes pool) cultured for 2 days on 15 mm diameter Alvetex Scaffold discs. (A) 12-well insert format: (i.) contact feeding by media from beneath; (ii.) submerged culture. (B) 24-well plate format. Cells were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin. Images acquired at 20× magnification.
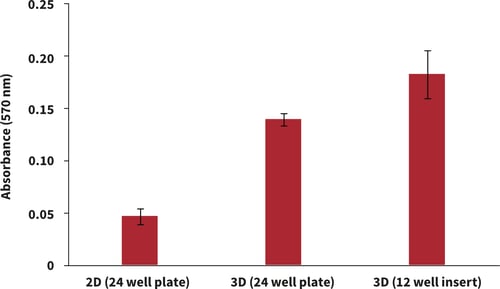
Figure 2. Biochemical analysis of cell viability using a standard MTT assay. Data from 2 replicate cultures of rat hepatocytes (Biopredic Int., HEP134, Male rat cryopreserved hepatocytes pool) are shown, each sampled in triplicate (n = 2, mean ± SD). Cells were cultured for 24 hours in 2D in 24 well plate (collagen coated), Alvetex Scaffold in 24 well plate (collagen coated), and Alvetex Scaffold in 12 well insert in 12 well plate (uncoated) formats.