The ultimate guide to REPROCELL’s GMP iPSC manufacturing service

What are GMP iPSCs?
GMP iPSCs are induced pluripotent stem cells (iPSCs) that can be used for therapeutic applications. These unique cells are generated from adult tissue biopsies reprogrammed into induced pluripotent stem cells, rather than being derived from embryonic tissues.
GMP iPSCs are typically used in a therapeutic setting, so they must undergo additional quality checks to ensure that they are fit for human use. Manufacture under GMP and adherence to guidelines established by the regulatory authorities ensures iPSCs of the highest quality.

REPROCELL provides stem cell services for cell therapy manufacturing.
What makes REPROCELL's stem cells different?
While a few companies offer iPSC seed-stock generation, REPROCELL provides the most comprehensive GMP iPSC manufacturing service. With expertise in primary tissue procurement, RNA reprogramming, and cell expansion, we eliminate the hassle of dealing with multiple companies – cutting down paperwork and redundant conversations.
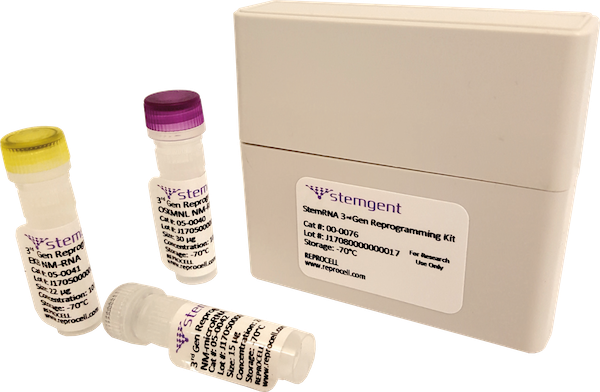
In addition, all our custom service projects are milestone-based – meaning we can tailor your project to suit your research and budget requirements – and we are the only company to offer StemRNA™ Reprogramming Technology for generating your GMP iPSC Master Cell Bank. RNA reprogramming is highly recommended for clinical applications because it is footprint and virus-free, producing cells that are genetically robust and stable. The manufacturing process is consistent with regulatory guidelines set by the FDA, PMDA, and EMA.
What are the benefits of choosing REPROCELL?
The key benefits of choosing REPROCELL for your GMP iPSC manufacturing include cells that are:
- Manufactured in accordance with the ICH 5QA process
- Generated using footprint-free RNA reprogramming technology
- Available for commercial use (with a fee)

iPSCs from donors that meet your criteria
At REPROCELL, we have unrivaled access to biological samples and over 30 years of experience in human tissue procurement. It is often difficult to have access to primary tissues from your desired patient population, we can locate relevant donors based on demographics and medical history.
iPSCs generated using DNA-free, RNA reprogramming technology
REPROCELL’s StemRNA™ Reprogramming Technology generates robust iPSCs that display low batch-to-batch variation without the need of screening for remaining exogenous genes and vectors.
iPSC seed stocks, Master Cell Banks (MCBs), and working cell banks (WCBs) from REPROCELL are available for commercial and therapeutic use with very reasonable terms.
Looking for a comprehensive GMP iPSC manufacturing service?
If you are looking to outsource a single company for your GMP Master Cell Bank, our global team can help you at any stage of therapeutic development. Our scientists have successfully completed hundreds of studies for clients, including 24 of the top 25 biotech and pharma companies. To find a solution for your clinical iPSC needs, contact us today.

REPROCELL provides stem cell services for cell therapy manufacturing.

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.