Primate ES Cell Medium
RCHEMD001
Brand: REPROCELL®
Serum-free formulation for feeder-dependent ES (embryonic stem) / iPS (induced pluripotent stem) cell culture.
Basic FGF (bFGF) needs to be purchased separately.
Currency:
| Product name | Catalog number | Pack size | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|
| Primate ES Cell Medium | RCHEMD001 | 500 mL | (select above) | $ 286.00 | £ 234.00 | € 274.00 |
Note: prices shown do not include shipping and handling charges.
Product Information
The gold standard culture medium for human ES/iPS cell culture on feeders
- Just add bFGF before use, if necessary. No other mixing required
- Each lot is functionally tested by suing human iPS cells
- Lot-to-lot control of other critical criteria including osmolarity, pH, sterility, and Mycoplasma
- Serum-free
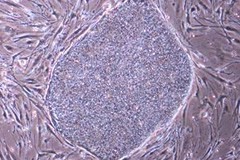
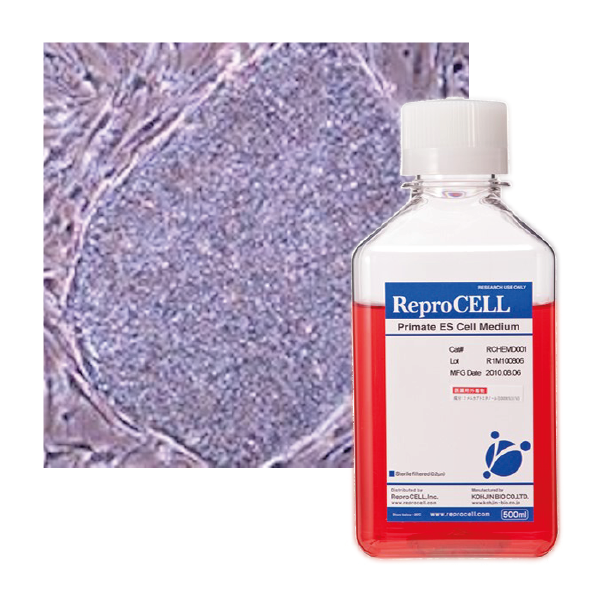
Human iPSCs cultured using Primate ES Cell Medium with 5 ng/mL bFGF on MEF cells.
REPROCELL’s Primate ES Cell Medium is a serum-free formulation for feeder-dependent ES (embryonic stem) / iPS (induced pluripotent stem) cell culture that was jointly developed by REPROCELL and Kyoto University. This stem cell media enabled development of the world’s first human induced pluripotent stem cells in 2007 by Prof Shinya Yamanaka and his colleagues. Following this breakthrough, REPROCELL’s Primate ES Cell Culture Medium has been successfully adopted for culture of stem cells by numerous labs throughout the world to promote a wide range of stem cell applications. This stem cell media has been specifically developed to simplify the process of ES and iPS cell culture for human and other primate cells. You just need to add bFGF prior to use* and the medium is ready for use.
* Not necessary for all the cell lines.
Basic FGF (bFGF) needs to be purchased separately.
The REPROCELL company name and logo are the property of REPROCELL Inc., Japan.
Product Name : Primate ES Cell Culture Medium
Catalog Number : RCHEMD001-A
Size : 500 mL
Storage and Stability: Store basal medium at –20 °C. Medium is stable for 3 years when stored as directed.
Quality Control:
- Each lot functionally tested using human iPS cells. (Takahashi K, et al., Cell, 131, 861–72, 2007)
- Lot-to-lot control of other critical criteria including osmolarity, pH, sterility and mycoplasma.
- Ready-to-use formulation — no mixing required.
- Serum free
Notice To Purchaser: This product is for research use only, not for therapeutic or diagnostic purposes. Commercial use or resale requires separate permission from REPROCELL.
Manual:
- Tsujisaka T; Hatani T; Okubo C; Ito R; Kimura A; Narita M; Chonabayashi K ;Funakoshi S; Lucena-Cacace A; Toyoda T; Osafune K; Kimura T; Saito H; Yoshida Y.. Purification of human iPSC-derived cells at large scale using microRNA switch and magnetic-activated cell sorting. Stem Cell Rep 17:1772 (2022).
- Nakazato T Kawamura T; Uemura T; Liu L; Le J; Sasai M; Harada A; Ito E; Iseoka H; Toda K; Sawa Y; MiyagawaS. Engineered three-dimensional cardiac tissues maturing in a rotating wall vessel bioreactor remodel diseased hearts in rats with myocardial infarction. Stem Cell Rep 17:1170 (2022).
- Yokoyama J; Miyagawa S; Akagi T; Akashi M; Sawa T. Human induced pluripotent stem cell-derived three-dimensional cardiomyocyte tissues ameliorate the rat ischemic myocardium by remodeling the extracellular matrix and cardiac protein phenotype. PLOS One 16:e0245571 (2022).
- D'Souza SS; Kumar A; Maufort J; Weinfurter JT; Raymond M; Strelchenko NS; Perrin E; Coonen J; Mejia A; Simmons HA ;Torbett BE; Reynolds M; Thomson JA; Slukvin II. Assessment of Safety and Immunogenicity of MHC homozygous iPSC-derived CD34+ Hematopoietic Progenitors in a NHP Model. Blood Advances :ADV-2022-006984R1 (2022).
- D'Souza SS; Kumar A; Weinfurter J; Park MA; Maufort J; Tao L; Kang J; Dettle ST; Golos T; Thomson JA; Reynolds MR; Slukvin I. Generation of SIV-resistant T cells and macrophages from nonhuman primate induced pluripotent stem cells with edited CCR5 locus. Stem Cell Rep 17:p953 (2022).
- Takeda M; Ito E; Minami K; Harada A; Mochizuki-Oda N; Sawa Y; Miyagawa S. Elimination of residual undifferentiated induced pluripotent stem cells (iPSCs) using irradiation for safe clinical applications of iPSC-derived cardiomyocytes. Biochem Biophys Res Commun 574:91 (2021).
- Kobayashi H; Hatakeyama H; Nishimura N; Yokota M; Suzuki S; Tomabechi Y; Shirouzu M; Osada H; Mimaki M; Goto Y-i; Yoshida M. Chemical reversal of abnormalities in cells carrying mitochondrial DNA mutations. Nature Chem BIol in press://doi.org/10.1038/s41589-020-00676-4 (2020).
- Kuroda K; Komori T; Ishabashi J; Uto T; Kobayashi I; Kadokawa R; Kato Y; Ninomiya K; Takahashi K; Hirata E. Non-aqueous, zwitterionic solvent as an alternative for dimethyl sulfoxide in the life sciences. Communications Chem 3:163 (2020).
- Hidaka T; Imamura K; Hioki T; Yakagi T; Giga Y; Giga M-H; Nishimura Y; Kawahara Y; Hayashi S; Niki T; Hushimi M; Inoue H. Prediction of Compound Bioactivities Using Heat-Diffusion Equation. Patterns in press://doi.org/10.1016/j.patter.2020.100140 (2020).
- Kase Y; Okano H. Expression of ACE2 and a viral virulence-regulating factor CCN family member 1 in human iPSC-derived neural cells: implications for COVID-19-related CNS disorders. Inflammat Regen 40:32 (2020).
- Samura T; Miyagawa S; Kawamura T; Fukushima S; Yokoyama J-y; Takeda M; Harada A; Ohashi F; Sato-Nisiuchi R; Toyofuku T' Toda K' Sekiguchi K; Sawa Y. Laminin-221 Enhances Therapeutic Effects of Human-Induced Pluripotent Stem Cell–Derived 3-Dimensional Engineered Cardiac Tissue Transplantation in a Rat Ischemic Cardiomyopathy Model. J Am Heart Assoc 9:e015841 (2020).
- Hambata T; Umeda K; Houzuki K; Tanaka T; Daifu T; Nodomi S; Saida S; Kato I; Baba S; Hiramatsu H; Osawa M; Niwa A; Saito MK; Kamikubo T; Addachi S; Hashii Y; Shimada A; Watanabe H; Osafune K; Okita K; Nakahata T; Watanabe K Takita J; Heike T. Pluripotent stem cell model of Shwachman–Diamond syndrome reveals apoptotic predisposition of hemoangiogenic progenitors. Sci Rep 10:14859 (2020).
- Sasaki B; Uemoto S; Kawaguchi Y. Transient FOXO1 inhibition in pancreatic endoderm promotes the generation of NGN3+ endocrine precursors from human iPSCs. Stem Cell Res in press:101754 (2020).
- Grajcarek J; Monlong J; Nishinaka-Arai Y; Nakamura M; Nagai M; Matsuo S; Lougheed D; Sakurai H; Saito MK; Borque G; Woltjen K. Genome-wide microhomologies enable precise template-free editing of biologically relevant deletion mutations. Nature Commun 10:4856 (2019).
- Takahashi M; Yamazaki S. Generation of a human induced pluripotent stem cell line, IMSUTi002-A-1, harboring the leukemia-specific fusion gene ETV6-RUNX1. Stem Cell Research 40: (2019).
- Takada H; Kaieda A; Tawada M; Nagino T: Sasa K; Oikawa T; Oki A; Sameshima T; Miyamoto K; Miytmoto M; Kokubu Y; Tozawa R; Sakurai H; Saito B. Identification of 2,6-Disubstituted-3H-imidazo[4,5-b]pyridines as Therapeutic Agents for Dysferlinopathies through Phenotypic Screening on Patient-Derived iPSCs. J Med Chem In press:1538 (2019).
- Umemura Y; Maki I; Tsuchiya Y; Koike N; Yagata K. Human circadian molecular oscillation development using induced pluripotent stem cells. J Biol Rhythms 34:525 (2019).
- Watanabe T; Yamazaki S; Yoneda N; Shinohara H; Tomioka I; Iiguchi T; Tagoto M; Ema M; Suemizu H; Kawai K; Sasaki E. Highly efficient induction of primate iPS cells by combining RNA transfection and chemical compounds. Genes to Cells in press:doi:10.1111/gtc.12702 (2019).
- Kuroda T; Yasuda S; Tachi S; Matsuyama S; Kusakawa S; Tano K; Miura T; Matsuyama A; Soto Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nature Commun 10:2175 (2019).
- Minami T; Ishii T; Yasuchika K; Fukumitsu K; Ogiso S; Miyauchi Y; Kojima H; Kawai T; Ramaoka R; Oshima Y; Kawamoto H; Kotaka M; Yasuda K; Osufune K; Uemoto S. Novel hybrid three-dimensional artificial liver using human induced pluripotent stem cells and a rat decellularized liver scaffold. Regenerative Therapy 10:127 (2019).
- Ohashi F; Miyagawa S; Yasuda S; Miura T; Kuroda T; Itoh M; Kawaji H; Ito E; Yoshida S; Saito A; Sameshima T; Kawai J; Sawa Y; Sato Y. CXCL4/PF4 is a predictive biomarker of cardiac differentiation potential of human induced pluripotent stem cells. Sci Rep 9:3638 (2019).
- Nazeki F; Tsuge I; Horie T; Imamura K; Tsukita K; Hotta A; Baba O; Kuwabara Y; Nishino T; Nakao T; Nishiga M; Nishi H; Nakashima Y; Ide Y; Koyama S; Kimura M; Tsuji S; Maitoh M; Suzuki S; Izumi Y; Kawarai T; Kaji T; Kimura T; Inoue H; Ono K. MiR-33a is a therapeutic target in SPG4-related hereditary spastic paraplegia human neurons. Clinical Sci 133:583 (2019).
- Cieslar-Pobuda A; Rafat M; Knoflach V; Skonieczna M; Hudecki A; Malechi A; Urasinska E; Ghavami S; Los MJ. Human induced pluripotent stem cell differentiation and direct transdifferentiatoin into corneal epithelial-like cells. Oncotarget 7:42314 (2016).
- Morizane R; Lam AQ; Freedman BS; Kishi S; Valerius MT; Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature Biotechnol 33:1193 (2015).
- Katayama S; Shimoda K; Yakenaga Y. Loss of ADAR1 in human iPS cells promotes caspase3‐mediated apoptotic cell death. Genes to Cells 20:675 (2015).
- Matsuura K, Wada M, Shimizu T, Haraguchi Y, Sato F, Sugiyama K, Konishi K, Shiba Y, Ichikawa H, Tachibana A, Ikeda U, Yamato M, Hagiwara N, Okano T. Creation of human cardiac cell sheets using pluripotent stem cells. Biochem Biophys Res Commun 425:321-7 (2012).