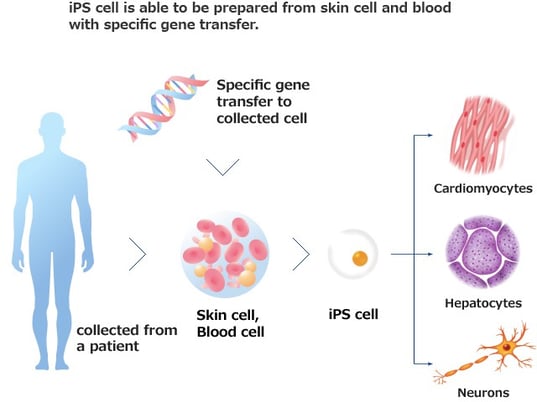
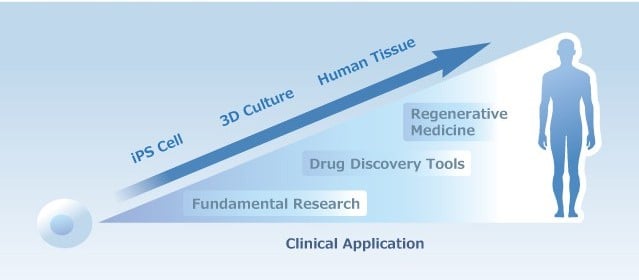
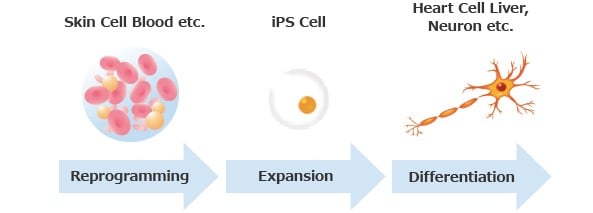
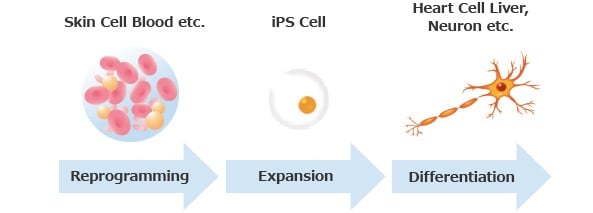
As a growing industry, we are supported by the government in Japan.
As part of its growth plan, the Japanese administration has pledged 110 billion yen over 10 years to support medical research using iPSCs. It has also established a “Japanese National Institutes of Health”, based on the USA’s NIH, and has revised the Pharmaceutical Affairs Law to approve medical technologies quicker.
REPROCELL’s headquarters is based in a special zone for iPSC Research
We are based in Yokohama City, Kanagawa Prefecture, which is part of the Life Innovation in Keihin Coastal Areas “Comprehensive Special Zones for International Competitiveness Development”". This zone aims to increase international competitiveness by allowing global businesses to lead medicine and medical equipment production, helping related industries and SMEs prosper.
The legislation in Japan supports regenerative medicine
Two laws came into effect on November 25, 2014. These not only clarified the standards and processes for regenerative medicine products but also accelerated the approval process:
- The “Law on Guaranteeing Safety in Regenerative Medicine” or “Regenerative Medicine Law”
- The “Law on Guaranteeing Safety, Efficacy, and Quality in Medical Supplies and Equipment”, also called the “Medical Supplies and Equipment Law”