Histology (4): Processing Alvetex® Scaffold 3D Cultures for Resin Embedding and Toluidine Blue Staining for Light Microscopy
1. Introduction
Following fixation, a variety of cytological stains can be used allowing different cell components to be visualised in detail. When structural integrity of sample architecture is paramount, samples are embedded in resin. Resin embedding allows ultrathin sections of samples to be obtained with no loss of structural detail.
Here we describe the use of toluidine blue as a stain of sectioned material for light microscopy. Toluidine blue is a general histology stain that, in basic solutions, binds to nucleic acids and all proteins. It is also commonly used for staining thin sections (0.5-1 μm) of resin embedded tissues to aid with the orientation and visualisation of samples subsequently prepared for electron microscopy. While everything in the sample stains with the dye, structural details remain highly prominent due to the thinness of the sections.
Alvetex Scaffold can easily be processed like a standard tissue sample, allowing established histology methods to be followed with excellent results. An example protocol is outlined below.
2. Materials and Methods
2.1. Materials Required
- Karnovsky’s fixative (2 % paraformaldehyde; 2.5 % glutaraldehyde in 0.1 M phosphate buffer pH 7.4. (for full recipe see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures.)
- 0.1 M phosphate buffer (for full recipe see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures.)
- Dehydration ethanols (30 %, 50 %, 75 %, 95 %, 100 %)
- 1 % buffered osmium tetroxide (1 % osmium tetroxide solution (Agar scientific Ltd), 0.1 M phosphate buffer (pH 7.4). (For full recipe detail see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures.)
- L R White Resin (Agar Scientific Ltd)
- 1 % Toluidine Blue in 1 % sodium borate
- DPX
- Basic equipment: spade forceps to handle 3D cultures, scalpel (to trim/cut Alvetex Scaffold cultures if required), disposable pipettes, embedding moulds, wire loop
2.2. Karnovsky’s and Osmium Fixation of Scaffolds
- Aspirate off the medium and carefully wash the 3D culture twice with PBS.
- Remove well inserts from cradle and carefully cut small samples (2-3 mm square) from the scaffold disc.
- Immerse samples in Karnovsky’s fixative at 4 °C for 90 minutes. The ratio of fixative volume to specimen size should be approximately 20:1.
- After fixation, aspirate the fixative into a waste container for disposal.
- Wash the specimen twice for 2 minutes in 3 ml 0.1 M phosphate to remove excess fixative, discarding waste liquid.
- Add 20× the specimen volume of 30 % ethanol. Leave to equilibrate for 5 minutes preferably on a rotating wheel. Pour off the ethanol and discard. Repeat twice.
- Repeat step 2.6 with 50 %, 75 %, 95 % and then absolute ethanol. The sample can be stored in absolute ethanol prior to resin embedding.
2.3. Resin Embedding and Sectioning
- Prepare the resin/catalyst mix according to the manufacturer’s instructions.
- Immerse samples in a 1:1 mix of absolute ethanol and L R White resin (20x the specimen volume) and leave on a rotor for 1 hour at room temperature.
- Replace the resin/ethanol mix with 3 changes of resin alone, for at least 60 minutes each. Samples can also be left in resin at room temperature overnight.
- Embed the specimen according the manufacturer’s instructions. Specimens may be embedded in gelatin capsules or flat embedding moulds made of PTFE.
- It is possible to cut LR White resin blocks on a standard microtome with a steel knife blade. The method of choice, however, is to use a motorized ultra-microtome and glass knife. For Toluidine Blue staining 1.0 µm sections should be cut.
- Place a drop of distilled water approximately 1 cm in diameter on a clean glass slide. Pick the cut sections up with a wire loop and invert the loop to place 2-4 sections into the drop of water on the slide.
- Place the slide with the water droplet containing sections on a hot plate adjusted to approximately 60-65°C and wait until all the water evaporates.
2.4. Toluidine Blue Staining
- Locate the sections on the slide and circle the bottom of the slide with a permanent marker to indicate their position.
- Place 1-2 drops of 1 % toluidine blue in 1 % sodium borate onto the sections and place the slide on the hot plate. Wait until the edges of the drop of stain begin to turn golden (approximately 20-30 seconds).
- Remove the slide and rinse the sections with a gentle stream of distilled water from a wash bottle to wash off the excess stain.
- Differentiate the sections by rinsing in a gentle stream of 50 % ethanol.
- Replace the washed slide on the hot plate after gently drying the bottom of the slide with a piece of paper towel and wait for the residual ethanol to evaporate from the slide surface.
- Remove the slide from the hot plate and add 1-2 drops of DPX mounting medium to the top of the sections adhering to the slide. Gently lower a coverslip onto the droplet of Polymount over the sections.
- Allow to dry and store in a dust free location.
3. Example Results
Figure 1 shows an example of a resin embedded 3D Alvetex Scaffold culture, sectioned and stained with Toluidine Blue.
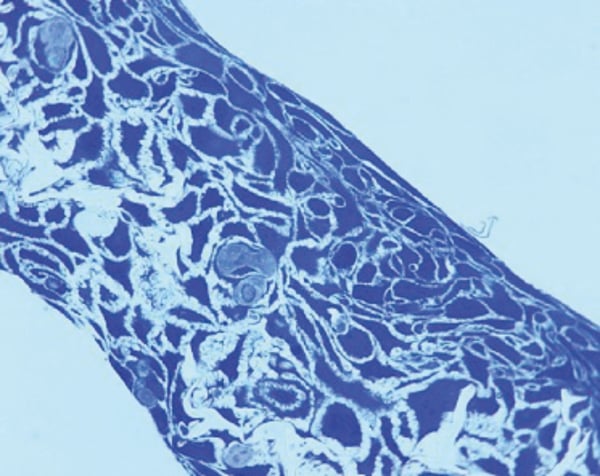
Figure 1. HaCaT cells grown in Alvetex Scaffold can be fixed and processed for histological analysis using standard methods. Sample fixed in Karnovsky’s, embedded in LR White resin, processed and counter stained with Toluidine Blue.
