Clinical Stem Cell Services
GMP MSC production service for your clinical application
REPROCELL and our experienced partner, Histocell, can help with your next clinical MSC project
Clinical Stem Cell Services
REPROCELL and our experienced partner, Histocell, can help with your next clinical MSC project
Mesenchymal Stem Cells (MSCs), also known as Mesenchymal Stromal Cells, have received a lot of interest, both as direct therapeutic agents and for their potential to differentiate into clinically relevant somatic cell types and tissues. More than 1000 clinical trials using MSCs in a wide variety of therapeutic areas have been reported.
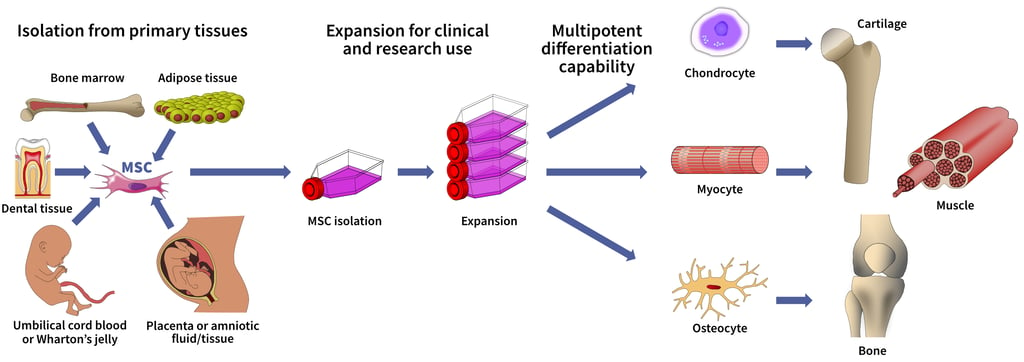
Use of MSCs for Clinical Projects. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissue, and cord blood or placenta. These MSCs can be purified and expanded in the laboratory to generate sufficient cells for development of a cell therapy. Expanded MSCs can be differentiated to a variety of tissues for therapeutic applications, including cartilage, muscle, and bone.
MSCs can be isolated from multiple sources and possess the ability for self-renewal and multi-lineage differentiation (above). Further, primary MSCs can migrate toward sites of injury and tumor microenvironments, making them attractive for use as a drug delivery vehicle for targeted therapies.
All factors secreted by MSCs including paracrine substances and exosomes (extracellular vesicles) can be isolated and harvested. The MSC secretome has been shown to promote immune modulation and tissue modulation during various applications in regenerative medicine therapy.
Note: we also have Research grade MSCs and products for MSC research.
Histocell is a CDMO and a clinical-stage biopharmaceutical company specializing in developing cell therapy products for regenerative medicine. Under their established and authorized GMP Advanced Therapy Medicinal Product (ATMP) manufacturing facility, they have been providing GMP manufacturing services since 2011. Around 300 patients have already been treated with ATMPs manufactured by Histocell, and more than 350 batches have been manufactured under GMP. A highly reliable and qualified scientific team with more than 20 years of experience will take care of your cell therapy needs, with a flexible and scalable manufacturing process.

We provide all regulatory QC specified by the EMA or FDA, and all the required documentary support for the cells.
Histocell owns a new Advanced Therapies GMP cell production unit with more than 600 m2 of capacity




R&D lab – GMP-like production and QC

Discover more
Resources
Gene Editing Services
