Stem cells offer hope for sufferers of Spinocerebellar Ataxia
What is Spinocerebellar Ataxia?
Spinocerebellar ataxias (SCAs) are a group of inherited, genetically heterogeneous disorders which are characterised by ocular motor abnormalities, cognitive dysfunction, peripheral neuropathy and progressive cerebellar ataxia. In general, the prevalence of SCA is between 2-7/100,000 individuals with more than 30 subtypes being identified, each caused by a mutation of a different gene.
PolyQ SCAs including SCA1, SCA2, SCA3, SCA6, SCA7 and SCA17 are caused by an extensive CAG sequence repeat which encodes for expanded polyglutamine residues within the ATXN3 gene. The only currently approved treatment option for patients suffering with this debilitating, progressive disease is Ceredist, however this only provides palliative treatment in reducing or relieving frequency and severity of symptoms. Stem cell therapy is offering hope, with evidence of potential efficacy being demonstrated in a phase I/II clinical trial using stem cells isolated and cultured from human adipose tissue.

REPROCELL provides stem cell services for cell therapy manufacturing.
Clinical Trials
To date there have been a limited number of clinical trials in SCA3 and no trials in SCA2, with the trials that have been completed in SCA3 only recruiting a small number of patients and often displaying inadequate study design[1]. Additionally, none of the studies offered a strong rationale for selecting the respective study compound.
Despite researcher’s best efforts, no curative therapy has been approved which can delay or halt the progression of SCAs. There is strong scientific premise for targeting the mRNAs of the mutant ATXNs, with a variety of candidate drugs with different mechanisms of action being proposed, however these still require rigorous pre-clinical studies in SCAs, and there have been no relevant human observations thus far.
Are Stem Cells the Answer?
Mesenchymal stem cells (MSCs) are capable of differentiating into various cell types including mesodermal, ectodermal and endodermal lineages, and can be administered allogeneically as they display low immunogenicity. They also display reparative effects through secreting a broad repertoire of trophic factors, and several pre-clinical studies in animals showed that MSC infusion could partially restore motor function in SCA mouse models[2].
Steminent Biotherapeutics
Steminent Biotherapeutics[2] is a Taiwan-based cell therapy company which has built a proprietary allogeneic platform with which it aims to develop a pipeline of pre-clinical and clinical stage therapeutic programs for the treatment of a range of diseases. This unique isolation technology platform enables the isolation, processing and production of Steminent’s highly purified product Stemchymal® from adipose tissues. One of these diseases is PolyQ SCA.
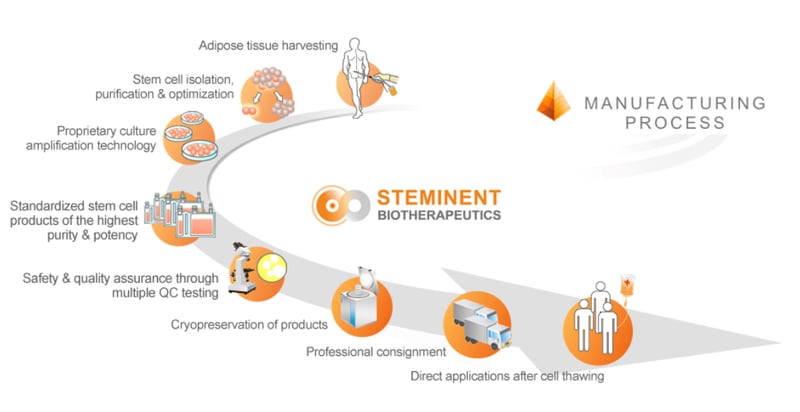
Steminent has completed a phase I/II clinical trial with Stemchymal® for the treatment of SCA with evidence of Stemchymal® safety generated in this trial with no biological-related adverse events observed in the 12-month follow up. Moreover, evidence of potential efficacy was observed in the preliminary Phase I/II data from two efficacy assessments: Scale for the Assessment and Rating of Ataxia (SARA) and the Sensory Orientation Test (SOT). Sixty-six percent of the subjects showed improvement in both the SARA score and the SOT score in the first month following a single infusion of Stemchymal®. These measured functional improvements were maintained in study subjects for up to 6 months.
Stemchymal® and REPROCELL
Steminent’s Phase II Stemchymal® SCA clinical program includes trials to evaluate safety and efficacy from treating patients in three countries. The first of these trials is currently enrolling patients in Taiwan, the FDA has approved a trial in the US, and REPROCELL are initiating a Phase II trial to assess Stemchymal® in treating PolyQ SCA patients in Japan.
Steminent announced last year that the Clinical Trial Notification (CTN) submitted by REPROCELL to the Pharmaceuticals and Medical Device Agency (PMDA) had been approved and this approval has allowed REPROCELL to move forward in preparing Japan trial sites and starting patient enrolment. This Phase II trial which aims to further demonstrate safety and efficacy, alongside the “Time-limited Conditional Approval” system should hopefully accelerate the development of regenerative products in Japan and offer hope to the approximately 30,000 people suffering from this debilitating condition.
If you want more information about participating in clinical trials, please contact your personal physician. They are the best person to provide information about treatment options for you.

REPROCELL provides stem cell services for cell therapy manufacturing.
References
- Trujillo-Martin et al. Effectiveness and safety of treatments for degenerative ataxias: a systematic review. Mov Disord 24 (2009)
- Jones et al. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model or cerebellar ataxia. Neurobiol Dis 40:2 (2010)
- Steminent Biotherapeutics - About Steminent

Author
Lindsey Moffitt, BSc
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.