MTT Cell Viability Assay of Cultures Grown on Alvetex® Scaffold in 3D
1. Introduction
This chromogenic assay involves the conversion of a yellow, water soluble compound, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) to a purple, insoluble formazan. This reaction only takes place when mitochondrial reductase enzymes are active, and therefore the conversion can be directly related to the number of viable (living) cells. The MTT reagent alone results in very low background absorbance values in the absence of cells on Alvetex Scaffold. It is recommended that for each cell type the linear relationship between cell number and signal produced should be first established in order to investigate the limits of the assay.
This is an example protocol, which may require further optimisation depending on the rate of cell growth of a particular cell type on Alvetex Scaffold.
2. Method
- Perform a PBS wash prior to running this assay if cells in Alvetex Scaffold were cultured in phenol red containing medium. If using the Alvetex Scaffold 12 well plate format, gently remove the discs using flat-ended forceps, gently dip in PBS and place into a new 12 well plate. If using well inserts, dip in PBS and then unclip the base of the insert to release the disc.
- Place each disc into a fresh 12 well plate and set up one well without Alvetex Scaffold as the negative control.
- Prepare 1 mg/mL MTT reagent (Sigma, M5655) solution in Phenol Red-free DMEM (Lonza, BE12-917F) and filter sterilise.
- Add 1 mL of MTT reagent to each well and cover with aluminium foil as it is light sensitive.
- Incubate at 37 °C and 5% CO2 for 1 hour.
- Prepare an acidified isopropanol solution (acidified isopropanol = 1 μL concentrated hydrochloric acid per 1 mL isopropanol).
- Aspirate off the MTT solution from each disc and replace by pipetting 1 mL of acidified isopropanol onto each disc plus the negative control.
- Cover plate in aluminium foil and place on rotating platform for 10 minutes at 100 rpm to ensure blue formazan product is fully solubilised.
- Pipette 20 μL of sample containing the formazan dye into a 96 well plate and add 180 μL of isopropanol to each well (This represents a 1 in 10 dilution of the formed product. Different dilutions may be required depending on the viable cell numbers expected.)
- Read absorbances at 570nm.
3. Example data set
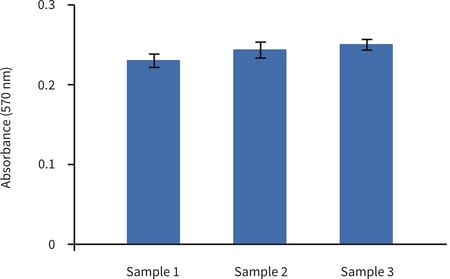
Figure 1. Biochemical analysis of cell viability using a standard MTT assay. Data from 3 sample replicates of HaCaT cells are show (n = 3, mean ± SD). Cells were cultured for 3 days on 22 mm Alvetex Scaffold discs presented in the 12 well plate format. Note good reproducibility between samples.
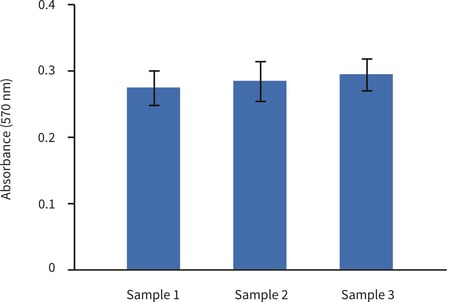
Figure 2. Biochemical analysis of cell viability using a standard MTT assay. Data from 3 sample replicates of HaCaT cells are shown (n = 3, mean ± SD). Cells were cultured for 3 days on 22 mm Alvetex Scaffold discs presented in 6 well inserts in 6 well plates. Note the good reproducibility between samples.
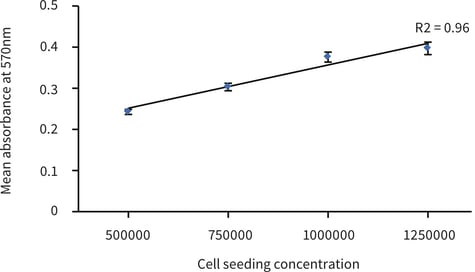
Figure 3. HaCaT cells cultured for 1 day on Alvetex Scaffold 22 mm discs in 12 well plate format
(AVP002; n = 3, mean ± SE). Good linearity was observed (R2 > 0.9) between cell number and signal
produced when cells were seeded at 0.5, 0.75, 1 and 1.25 × 106 viable cells per well as determined
by the Trypan Blue exclusion assay.
