Example Protocol for the Co-Culture of the Caco-2 Cell Line with the CCD-18co Cell Line on Alvetex® Scaffold in Well Insert Formats
1. Introduction
Caco-2 is a human colorectal adenocarcinoma cell line isolated in the late 1970s from colon carcinoma tissue[1]. Although Caco-2 cells have been used to model colon cancer cytotoxicity[2], they are better known for their ability to differentiate into functional enterocytes in vitro, characterised by the presence of tight junctions, a brush border and brush-border-associated hydrolases[3]. This has led to their widespread use as in vitro models of intestinal drug absorption[4] and bacterial or viral gut infection[5,6]. CCD-18co is a human fibroblast cell line isolated from normal colon tissue. Being a non-transformed cell line, CCD-18co is a popular choice as a normal control in cancer studies[7,8] and also to form a fibroblastic compartment in co-culture models of epithelial-stromal interactions in colon cancer[9]. In their own right, CCD-18co cells are also used to investigate mechanisms of pathological fibrosis following colon inflammation[10,11].
However useful mono-cultures of epithelial have been and continue to be in research and industry, it cannot be ignored that, in in vivo mucosal tissues, cross-talk between the cells of the epithelium and the fibroblasts within the underlying lamina propria is involved in a number of biological processes, ranging from normal development (uro-genital tract, breast[12]) to wound healing (lung[13]), as well as cancer (breast[14], colon[15] and prostate[16]). In this example protocol, we describe an in vitro mucosal co-culture model, by using the human colon carcinoma cell line Caco-2 as a collagen-supported confluent monolayer in combination with CCD-18co fibroblasts cultured within the depth of Alvetex Scaffold.
Alvetex Scaffold is available in several cell culture formats including 24 well plate (AVP006), 12 well plate (AVP002), 6 well insert (AVP004), 12 well insert (AVP005), and 24 well insert (AVP012).
24 well and 12 well plates are suitable for shorter term cultures and for applications where limited cell penetration into the scaffold is required. Well insert formats generally support longer term cultures and deeper cell penetration into the scaffold. They also provide for conveniently tailored media set ups (see the Alvetex Scaffold Quick Start protocol).
The availability of two different well insert formats enables choice on the basis of desired culture size and cell expenditure. 6 well inserts can be placed in conventional 6 well plates, while 12 well inserts can be placed in either 6 well plates or 12 well plates, depending on media requirements. Alternatively, both insert types can be housed in the dedicated Well Insert Holder in Deep Well Petri Dish (AVP015) to allow for increased media volumes and prolonged cell culture.
2. Methods
2.1. Preparation for 3D Cell Culture on Alvetex Scaffold
- Caco-2 cells were routinely maintained in T-75 flasks and CCD-18co are routinely maintained in T-225 flasks.
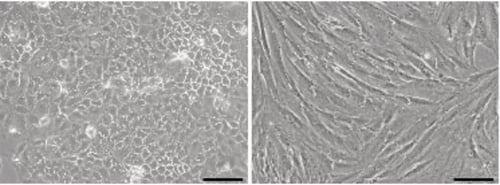
Figure 1. Phase contrast micrographs of Caco-2 cells (left) and CCD-18co cells (right) grown in conventional 2D culture plates. Images show cells at high confluency. Scale bars: 200 µm.
- For both Caco-2 and CCD-18co cells, complete growth media consisted of: high-glucose (4.5 g/L) DMEM supplemented with 10 % heat-inactivated FCS, 0.1 mM non-essential amino acids, 2 μm L-glutamine, 100 U/mL penicillin and 100 U/mL streptomycin.
Note: The complete growth medium described here is different from that described for Caco-2 monoculture (i.e. DMEM High glucose supplemented with 20 % v/v FBS, 0.1 mM non-essential amino acids, 10 mM HEPES buffer, 2 μm L-glutamine and 100 U/ml Penicillin/Streptomycin) to accommodate the needs of both cell types during co-culture.
- Cells were harvested by trypsinisation and centrifuged for 5 minutes (1000 rpm). The supernatant was discarded and the cell pellet was re-suspended in an appropriate volume of media for cell counting by Trypan Blue.
- Caco-2 cells were re-suspended at a concentration of 0.5 × 106 cells/mL for seeding. CCD-18co cells were resuspended at a concentration of 5.0 × 106 cells/mL for seeding.
Note: CCD-18co cells are seeded on Alvetex Scaffold 14 days prior to seeding Caco-2 cells.
2.2. 6 well Insert Format, AVP004
-
Alvetex Scaffold 6 well inserts in 6 well plate format were prepared for seeding by dipping in 70 % ethanol and washed twice with media (7 mL per well).
-
200 μL of the CCD-18co cell suspension was added in the centre of the Alvetex Scaffold disc, which was equivalent to 1 × 106 cells per well.
-
The plate was incubated for a minimum of 15 minutes at 37 °C with 5 % CO2 to allow the cells to settle into the scaffold.
-
Complete growth medium was added to each well to a total volume of 7 mL taking care not to dislodge cells from Alvetex Scaffold, i.e. feeding separately from above and below. Plates were re-incubated and medium was added to a full complement of 10ml per well the following morning.
-
Plates were maintained for a further 14 days by complete medium exchange once a week.
-
After 14 days, medium was removed down to an air-liquid interface, i.e. feeding from below only, and 300 μL of 2 mg/mL rat tail collagen I solution (Corning, cat # 354236, procedures may be found at https://ecatalog.corning.com/life-sciences/b2c/UK/en/Surfaces/Extracellular-Matrices-ECMs/Corning%C2%AE-Collagen/p/354236) were added to the surface of each insert. Plates were re- incubated for 3 hours to allow the collagen gel to set, after which fresh medium was added up to 10 mL per insert and plates were re-incubated overnight.
Note: A detailed protocol describing how to coat the top surface of Alvetex Scaffold with collagen I can be found at Alvetex protocols.
-
The following day, medium was removed down to air-liquid-interface and 1ml of Caco-2 cell suspension was added in droplets over the surface of the Alvetex Scaffold disc, which was equivalent to 0.5×106 cells per well.
-
Plates were incubated overnight at 37 °C with 5 % CO2 to allow the cells to settle on top of the scaffold.
-
The following morning complete growth medium was added to each well to a total volume of 10 mL taking care not to dislodge cells from Alvetex Scaffold.
Note: This method can be applied to the use of Alvetex Scaffold in 12 well insert format, AVP005. Adjust cell seeding and media volumes according to the guidelines provided in the Alvetex Scaffold Quick Start protocol.
- Plates were re-incubated and maintained for a further 5 days by complete medium exchange after every 2-3 days.
3. Example Data
3.1. 6 well insert format, AVP004
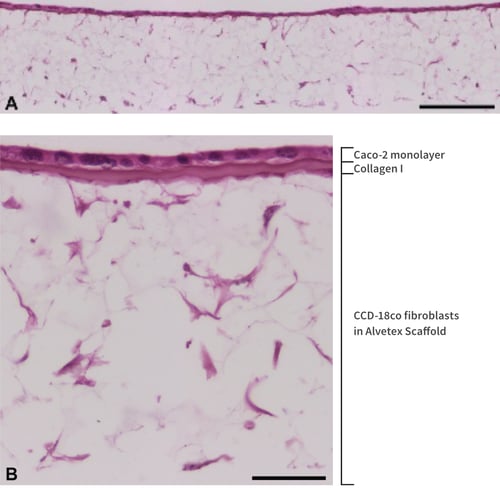
Figure 2. Figure 2. Low (A) and high (B) magnification brightfield micrographs of an even monolayer of Caco-2 cells form at the collagen-coated top surface of Alvetex Scaffold, with CCD-18Co fibroblasts underneath the same collagen layer and distributed throughout the depth of Alvetex Scaffold. CCD-18Co fibroblasts were grown on 22 mm diameter Alvetex Scaffold discs presented in 6-well insert in 6-well plate format for 14 days prior to layering with Collagen I and seeding of Caco-2 cells. Co-cultures were grown for a further 5 days, after which they were fixed, embedded in paraffin wax, sectioned (10 μm) and counterstained with haematoxylin and eosin. Scale bars: 200 µm (A) and 50 µm (B).
4. References
- Fogh J et al, 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59(1): 221-6.
- Viraq P et al, 2012. Superior cytotoxicity and DNA cross-link induction by oxaliplatin versus cisplatin at lower cellular uptake in colorectal cancer cell lines. Anticancer Drugs 23(10): 1032-8.
- Chantr et I et al, 1988. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res 48(7): 1936-42.
- Balimane PV et al., 2006. Current industrial practices of assessing permeability and P-glycoprotein interaction. AAPS J 8(1):E1-13.
- Gaillard JL et al, 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun 55(11): 2822-9.
- Svensson L et al, 1991. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol 65(8):4190-7.
- Grifin C et al, 2011. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. J Mol Cancer Ther. 10(1): 57-68.
- Takawa M et al, 2011. Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker. Cancer Sci. 102(7): 1298-305.
- Koshida Y et al, 2006. Interaction between stromal fibroblasts and colorectal cancer cells in the expression of vascular endothelial growth factor. J Surg Res. 134(2):270-7.
- Koon HW et al, 2010. Substance P modulates colitis-associated fibrosis. Am J Pathol. 177(5): 2300-9.
- Zhu Y et al, 2012. iNOS signaling interacts with COX-2 pathway in colonic fibroblasts. Exp Cell Res 318(16): 2116-27.
- Cunha GR et al, 2004. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 67(5): 417-34.
- Horowitz JC and Thannickal VJ 2006. Epithelial-mesenchymal interactions in pulmonary fibrosis. Semin Respir Crit Care Med 27(6): 600-12.
- Parrinello S et al, 2005. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 118(3): 485-96.
- Bosman FT et al, 1993. Epithelial-stromal interactions in colon cancer. Int J Dev Biol 37(1): 203-11.
- Niu YN and Xia SJ 2009. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl 11(1): 28-35.
Sticky sidebar content goes in here.
