IBD culture study Crohn’s or ulcerative colitis (UC)
Drug Discovery Assay – reference number: B106
Overview
| Tissue: | Human GI Mucosal Explants (IBD) |
| Target: | p38 MAP Kinase |
| Control Compound: | BIRB796 |
| Study Type: | Ex vivo Cultures |
| Functional Endpoint: | Inflammation |
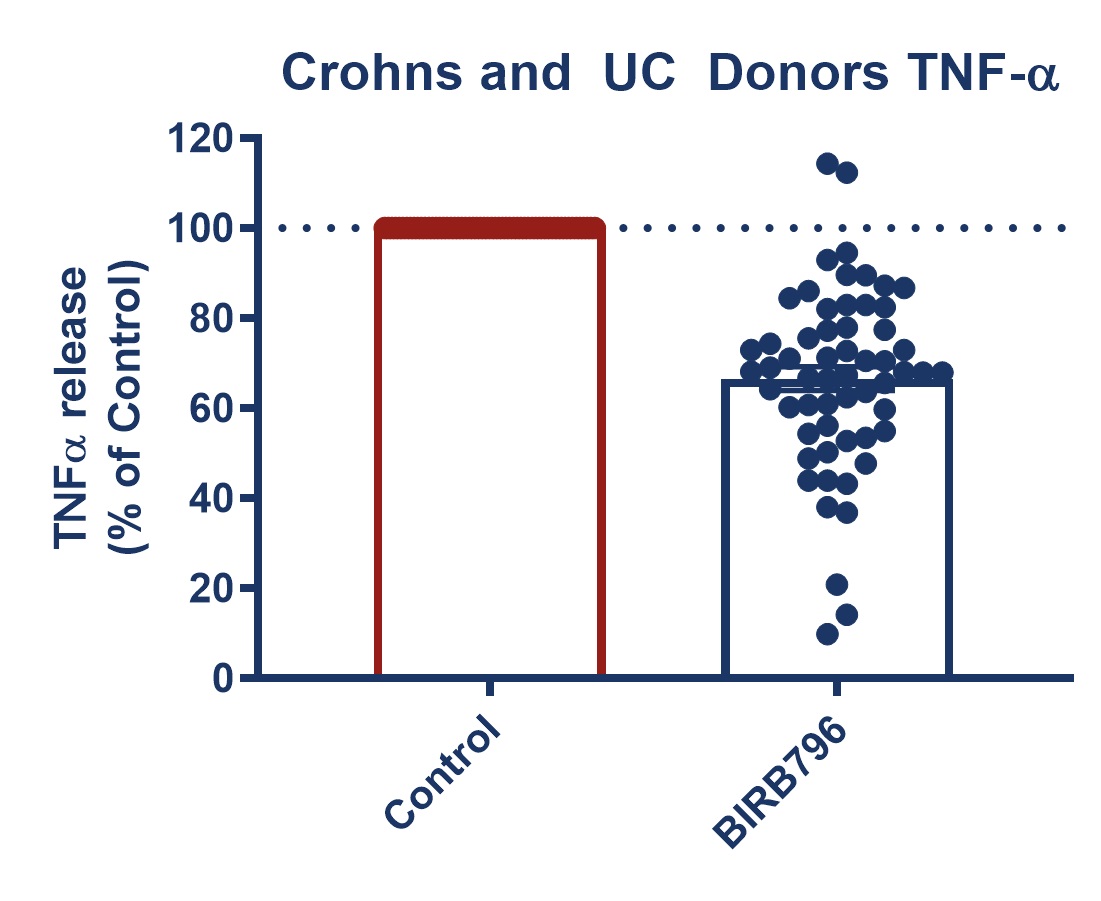
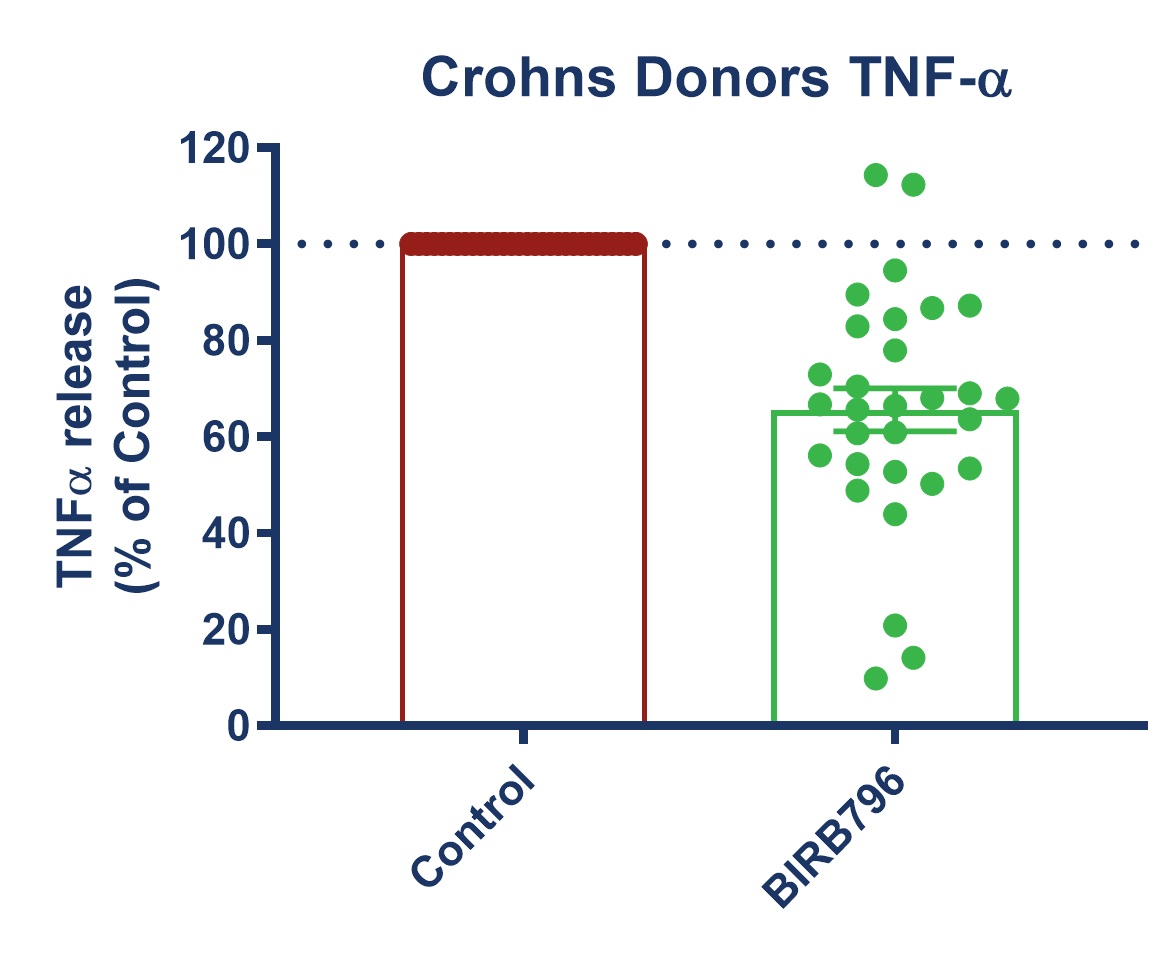
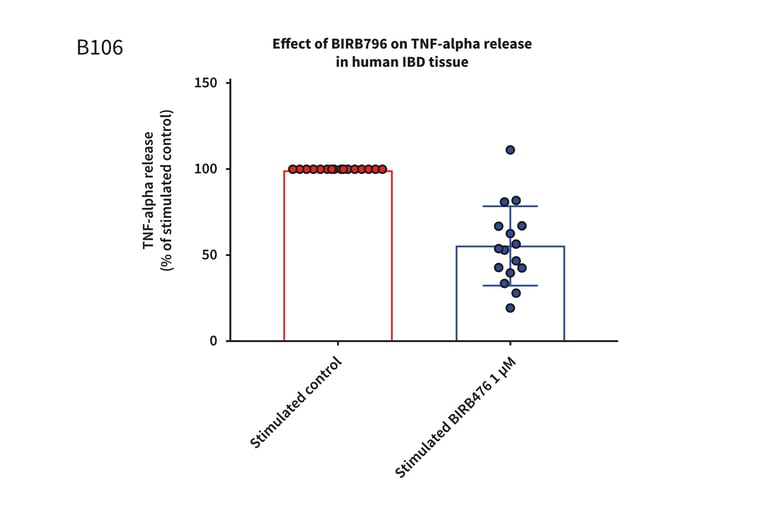 Figure 1: Graph demonstrating a reduction in TNF-alpha release in stimulated full thickness mucosal biopsies from Crohn’s and UC donors. Mucosal biopsies donated by 16 IBD patients were used to create a phenotypically relevant model of the disease. BIRBR796 was used as a reference compound, and the molecular target was p38 MAP Kinase. Analysis revealed a mean reduction in TNFα release in stimulated biopsies from Crohn’s and UC donors of 56.49% ± 5.76 (mean ± S.E.M).
Figure 1: Graph demonstrating a reduction in TNF-alpha release in stimulated full thickness mucosal biopsies from Crohn’s and UC donors. Mucosal biopsies donated by 16 IBD patients were used to create a phenotypically relevant model of the disease. BIRBR796 was used as a reference compound, and the molecular target was p38 MAP Kinase. Analysis revealed a mean reduction in TNFα release in stimulated biopsies from Crohn’s and UC donors of 56.49% ± 5.76 (mean ± S.E.M).
Key benefits summarised
- More than 40 inflammatory mediators can be measured
- Data from healthy, Crohn’s, and ulcerative colitis patients can be compared
- Cross-species comparison studies, e.g. with other preclinical models of IBD
- Tissue cytokine profiles correlate well with literature from clinical studies
- End-point analysis includes multiplex ELISA/Luminex assay, gene expression (RT-PCR), or immunohistochemistry (IHC)
 Microscope histological image of IBD colitis intestine
Microscope histological image of IBD colitis intestine
Testing Information
Assay Description
This experiment assesses whether test articles cause a reduction in TNF-alpha release with BIRBR796 as a reference compound. The specific results that will be provided are the effects of test articles on the levels of TNF-alpha release in human IBD tissue.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in distilled water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
A minimum of triplicate per condition with a maximum of 18 biopsies in total.
Study Outline
Rationale and Experimental Design
Full-thickness gut mucosal biopsies are dissected from fresh Crohn’s or UC tissue samples. Biopsies are taken at a size of 5mm2 and transferred to a plate containing specially fortified media before being incubated in optimum conditions for a maximum of 24 hrs. Biopsies are cultured in the presence of an inflammatory stimulant to aid in the normalisation of cytokine levels.
An example of the conditions assessed for 3 test articles are detailed below (it is recommended a minimum of triplicates should be used for each condition):
- Non-stimulated control
- Stimulated control
- Test article 1
- Test article 2
- Test article 3
- Positive control
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue.
End Point Analysis
Cytokine analysis: Supernatant samples can be analysed for specific analytes of your choice by a multiplex ELISA platform. Each analyte will be quantified by interpolation against a standard curve generated on the same 96 well analysis plate.
Other forms of endpoint analysis are available such as gene expression or immunohistochemistry.
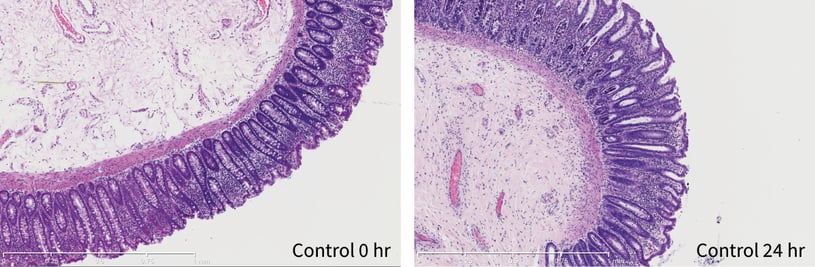
Figure 2: Colon explant histopathology maintained over a 24 hour period. At 0 hour good crypt morphology with intact lamina propria and muscularis mucosa can be sees. After 24 hours crypt morphology and muscularis mucosa were maintained. Slight degradation of epithelial structure but generally intact.
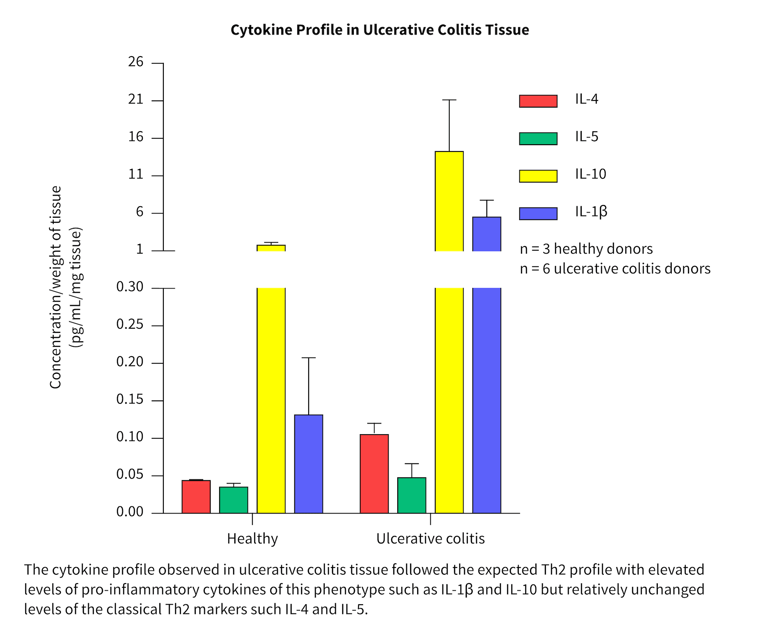
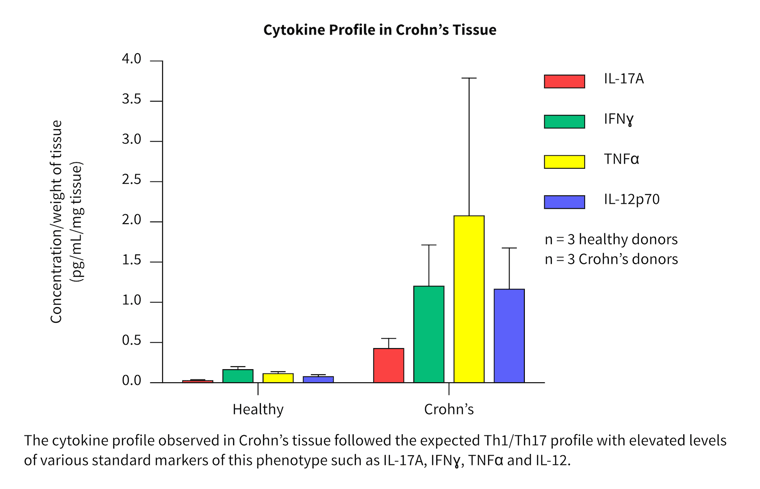
Cytokine profiles in normal and diseased tissue
- More than 40 cytokines/chemokines were measured in a pilot study to investigate the cytokine release profiles in healthy and diseased tissue.
- Biopsies from three healthy, three Crohn’s and six ulcerative colitis patients were tested and compared.
- Differences in the cytokine profile from each disease group were observed.
- Both disease groups showed separation from the healthy group for a range of key inflammatory cytokines and chemokines.
The cytokine profiles observed correlated well with literature from clinical studies.
Download our Explants of Inflammatory bowel disease tissue in culture poster (PDF)→
Clinical donor-donor and interdonor variation replicated ex vivo
 |
 |
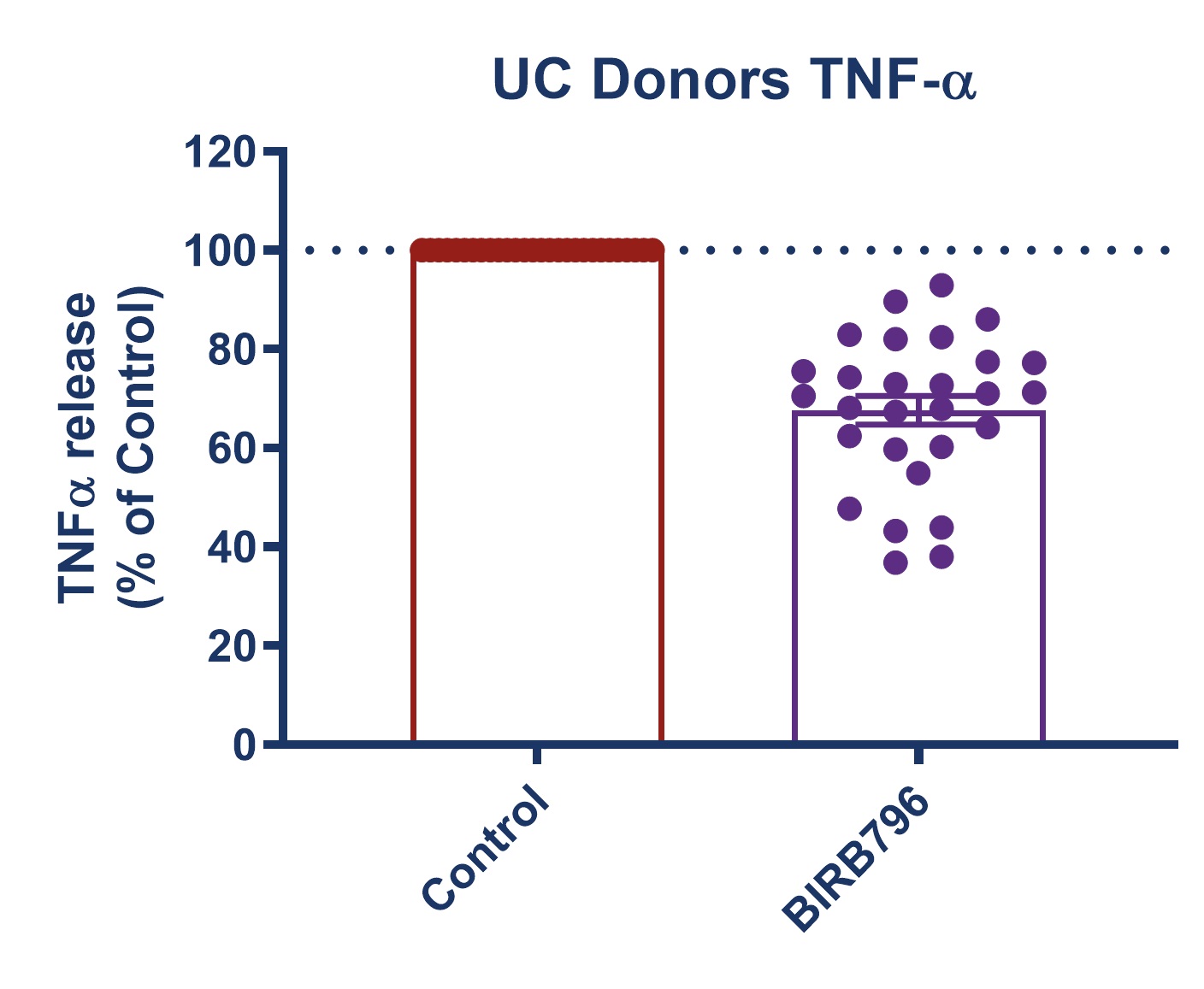 |
| Figure 3: Response to BIRB796 - TNFa Release in SEB-stimulated biopsies. On all graphs scatter dots represent the mean TNFα release for each individual donor. Bar/Line + error bars represent the mean ± SEM. | ||
FAQs
What is included in your IBD predictive drug discovery services?
- A customized project to meet your research goals.
- An expert Study Director assigned to manage each project as your single point of contact from initiation to report.
- Rapid access to human healthy and diseased tissues, through our industry-leading human tissue network.
- GLP projects are available.
- Ownership of all data generated during the project.
What is the estimated turnaround time for these studies?
For an IBD culture study requiring three donors, the estimated turnaround time is six weeks. This timeline is a guide based on REPROCELL’s standard tissue criteria and may be altered by client-imposed deviations.
What are the test agent requirements?
We ask that the test agent is provided in storable aliquots at the required test concentrations. Information should be provided on the diluent vehicle used, or else stock solutions are prepared in distilled water. The sponsor is also asked to provide a sufficient quantity of the test agent to run the entire study.
Are there exclusion criteria for donated tissues?
There are no specific exclusion criteria in place; however, donated tissues that are macroscopically diseased or necrotic will be rejected.
How are tissues standardised and qualified?
All biopsies are prepared at a standard size of approximately 5mm2 and can be weighed post experimentation. Due to the time limitations of using the tissues when fresh, tissues are qualified post-experimentation.
What conditions do you recommend for ex vivo culture studies?
It is recommended a minimum of triplicates should be used for each condition. An example of the conditions assessed for three test agents would be as follows:
- Non-stimualted control
- Stimulated control
- Test agent 1
- Test agent 2
- Test agent 3
- Positive control
How do you perform the end-point analysis?
Supernatant samples can be analysed for specific analytes of your choice by a multiplex ELISA platform. Each analyte will be quantified by interpolation against a standard curve generated on the same 96 well analysis plate. Other forms of end-point analysis are also available, such as gene expression or immunohistochemistry.




![Wire myography: the ultimate guide [protocol included]](https://www.reprocell.com/hs-fs/hubfs/REPROCELL-04.06.18_0163.jpg?width=756&height=425&name=REPROCELL-04.06.18_0163.jpg)