The ultimate guide to Alvetex
When Professor Stefan Przyborski invented Alvetex, he didn't just want to elevate 3D cell culture: he wanted to engineer in vitro models that mimicked the structure and function of living human tissues. The quest for improved translatability is the driving force behind the Przyborski lab, located in Durham University. In this ultimate guide to Alvetex, we will discuss the philosophy behind this unique approach to cell culture, and describe the most recent applications of Alvetex in ADME DMPK studies, skin cancer research, and more.
What is Alvetex?
Alvetex is a porous polystyrene membrane that can be used to culture cells in three dimensions. It is an inert, consistent structure that is 90% porous, providing maximal space for cell growth. Alvetex is available in a variety of formats, including multi-well plates and inserts.
Two versions of Alvetex are currently available:
- Alvetex Scaffold – our market leading product, is primarily designed for three dimensional culture of dissociated mammalian cells within the scaffold. Average void size: 42 μm.
- Alvetex Strata – our second generation product, is primarily designed to support the growth of cells and intact tissues on the surface of the membrane. Average void size: 15 μm.
This blog post is about the use of Alvetex Scaffold for generating models for generating in vitro 3D cell assays.
Alvetex Scaffold models discussed in this post:
If you want to find out how Alvetex Scaffold can be used for your cell culture experiments, you can access a full range of Alvetex protocols as well as Alvetex whitepapers and application notes on the REPROCELL website.
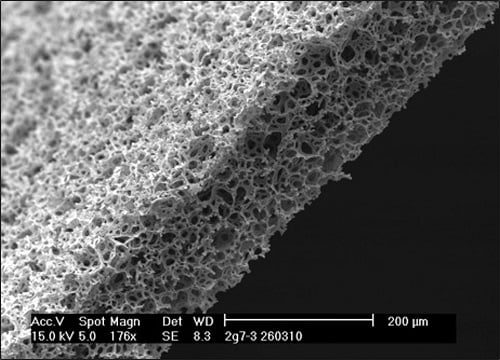

Figure 1: Alvetex Scaffold inert polystyrene membranes provide a platform to enable routine 3D cell growth. (Left) Empty Alvetex scaffold engineered into a 200-micron thick membrane. (Right) populated with cells to create a three-dimensional cell culture.
2D vs 3D cell culture
It is well accepted that the in vitro microenvironment can significantly alter experimental outcomes. In two-dimensional (2D) cell culture, cells are cultured in a basic environment on the bottom of plastic dishes, which can alter cell structure and behaviour.
Applying three-dimensional (3D) cell culture technologies establishes a more natural environment, enhancing the translatability of cell structure and function in vitro. For example, Alvetex causes cells to resemble real tissues by creating a structure where cells can populate and form interactions with one another.
This has a number of benefits for researchers, including:
- Advancement of the research and discovery process
- Improvement of cell culture models (providing more accurate data)
- Replacement and reduction of animal use in research
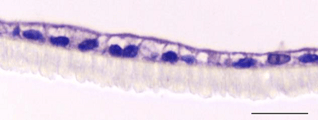
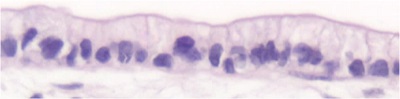
Figure 2: Two-dimensional cell culture of caco-2 cells (left) compared with three-dimensional cell co-culture of caco-2 cells (right)
Bioengineering epithelial tissues with Alvetex
If you are interested in constructing complex epithelial tissues in vitro, Alvetex can be used to achieve this. In our Alvetex models, the epithelium sits on top of a stromal layer of tissues, mostly comprised of fibroblasts, which provides nutritional and physical support. There are different types of epithelia of course in the body which have different functions, including:
- stratified keratinized (skin)
- simple columnar (intestine)
- stratified non-keratinized (oral mucosa)
- ciliated pseudostratified (nasal mucosa)
Alvetex Scaffold can be used to model all of these – discover:
Bioengineering epithelial tissues in vitro →
Stratified keratinized epithelium
The skin is the largest organ in the body and the most abundant source of stratified keratinized epithelium. The epidermis, which consists of stratified keratinized epithelium with an overlying stratum corneum, is supported by a dermal compartment. The dermis consists of multiple cell types but is primarily populated by fibroblasts and extracellular matrix (ECM). Because there is a broad range of cells and layers that make up the skin, it is difficult to model this complex organ in vitro using 2D culture.
With Alvetex, you can mimic the layered, multicellular structure of skin observed in vivo. This is achieved by populating the membrane with primary dermal fibroblasts for 28 days prior to adding keratinocytes. During this culture period, the fibroblasts deposit ECM throughout the Alvetex scaffold as they do in vivo. This means that by day 28, there is no need to add ECM artificially to your model: the fibroblasts have done the work for you. You can observe the accumulation of these ECM components using immunostaining or hydroxyproline assay (figure 3).
This initial culture period creates a foundation for seeding keratinocytes, which form the epidermis. In Alvetex, human primary keratinocytes can be maintained at the air-liquid interface for up to six months or more.
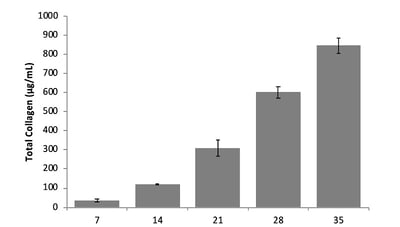
Figure 3: Hydroxyproline assay quantifies collagen within the Alvetex scaffold from 7-35 days.
Characterization via TEM
When compared with human biopsies, this model expresses the same epidermal differentiation markers, intracellular junctions, and ECM as real human skin. Transmission electron microscopy (TEM) analysis shows intercellular desmosomes between keratinocytes, hemidesmosomes along the entire basement membrane, and corneo-desmosomes within the stratum corneum (figure 4). The presence of lipid droplets in the upper epidermis and the flattened appearance of the corneocytes indicates terminal differentiation within the stratum corneum.
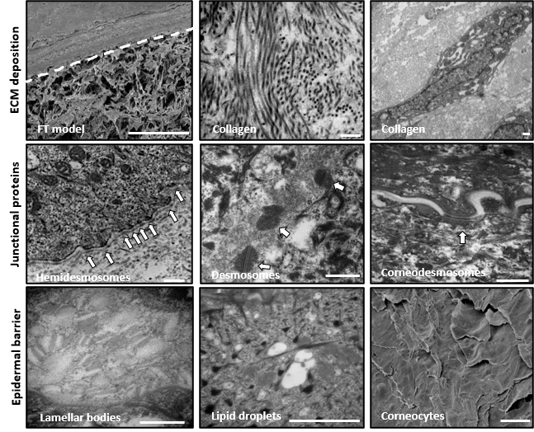
Figure 4: Fibroblasts secrete endogenous extracellular matrix organized in a transverse and longitudinal manner, similar to in vivo skin. (Top) Accumulation of collagen fibrils produced by fibroblasts and the ECM is all provided by the fibroblasts (Middle) Junctional complexes between cells: desmosomes, hemidesmosomes, and corneal desmosomes (Bottom) other ultrastructural features
Examples of customized Alvetex skin models
A range of skin states and diseases can be modeled using this in vitro system, which recapitulates the anatomy and physiology of real skin. The model is robust, reproducible, and can be maintained over extended periods of time, making it particularly useful for commercial applications.
Our scientists have partnered with major companies interested in developing skin health care products to create systems for measuring pigmentation, cancer, and aging of the skin. We have listed a few examples of these models and their experimental uses below.
Modeling skin pigmentation with Alvetex
A pigmented skin model can be created by introducing melanocytes into the in vitro skin assay. These melanocytes locate themselves at the basal area of the epidermis, just as they would in vivo (figure 5). By exposing these models to UV light (which gives them a visible suntan) we can study how the skin protects itself from exogenous stresses.
Under a microscope, we could observe melanocytes giving up their melanosomes to the surrounding keratinocytes in response to UV exposure. These melanosomes sit upon the cell nuclei, causing a supranuclear cap, which is how melanin protects DNA from UV radiation in vivo.
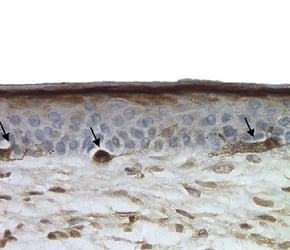
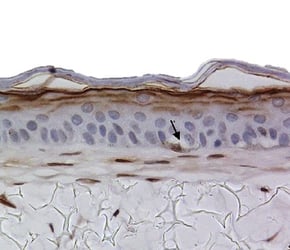
Figure 5: Melanocytes appear in the basal area of the model (indicated by arrows), staining positive for S100 marker.
Modeling skin cancer with Alvetex
Skin tumor cells can be introduced into this system to model malignancies such as melanoma. Below, we have introduced a pocket of cells into the dermal-epidermal junction of the model and then tracked their migration. We could then use this model to test drugs designed to suppress the invasion or proliferation of the tumor cells.
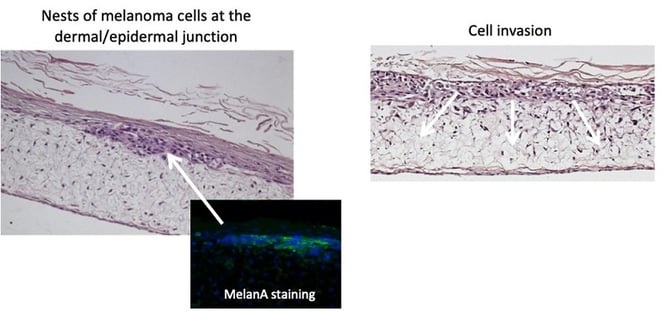
Figure 6: When melanoma cells are added to the Alvetex skin model, they can be seen migrating into the deeper layers of the tissue.
Modeling skin aging with Alvetex
As skin ages, collagen is lost from the dermal compartment and the epidermis becomes thinner - we have recapitulated this process in vitro using skin cells from aged donors. Skin aging can also be simulated by introducing senescent cells and studying the bystander effect that stimulates this phenotype. This technology is particularly useful for researchers developing anti-aging products.
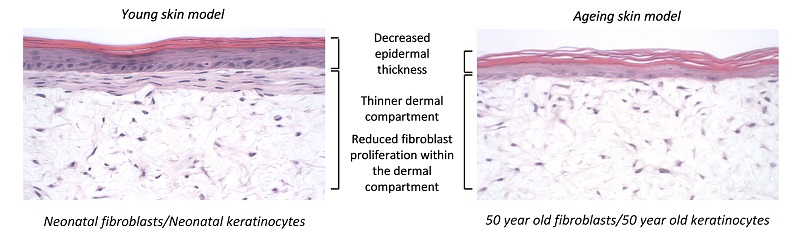
Figure 7: The Alvetex skin model can be used to mimic the aging process by using skin from aged donors or adding senescence cells.
Simple columnar epithelium
The lining of the gastrointestinal tract is vast and consists primarily of simple columnar epithelium. In the intestine, this epithelial lining sits on top of the lamina propria in the lumen of the gut wall. Caco-2 cells are often cultured on transwell membranes to mimic the intestinal wall, however, in this 2D environment, cells become flattened and do not behave as they would in vivo.
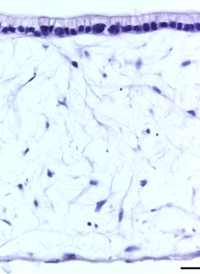
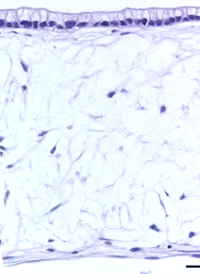
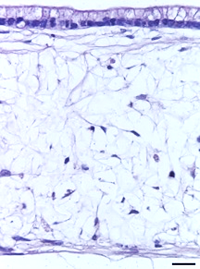
Using Alvetex, the in vivo environment of the gut lining can be recreated more accurately. By seeding the Alvetex scaffold with intestinal fibroblasts, a supportive foundation of ECM can be created for the caco-2 cells, which are subsequently added to create a columnar epithelium. In this microenvironment, the caco-2 cells become polarized (like their native counterparts) and no longer look like they do in a trans-well. After this point, the model can be maintained for up to 35 days and beyond.
| 14 days | 21 days | 28 days | 35 days |
 |
 |
 |
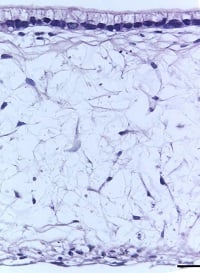 |
| Figure 8: Under the microscope, the columnar epithelium can be seen clearly on the top of the lamina propria. H&E staining 14, 21, 28, and 35-day sub-mucosal compartment with a 14 day Caco-2 monolayer seeded on top. Scale bar: 100 µm | |||
Characterization via TEM
This intestinal model can be characterized using TEM and compared with real intestinal tissue to ensure the anatomy is consistent. At high magnification, you see you can see the microvilli and the glycocalyx: important features of the intestinal epithelium. Ultrastructural analysis shows very nicely the tight junctions between the cells at the apical membrane, the microvilli, and the basement membrane which exists at the basal layer between the lamina propria and the epithelium.
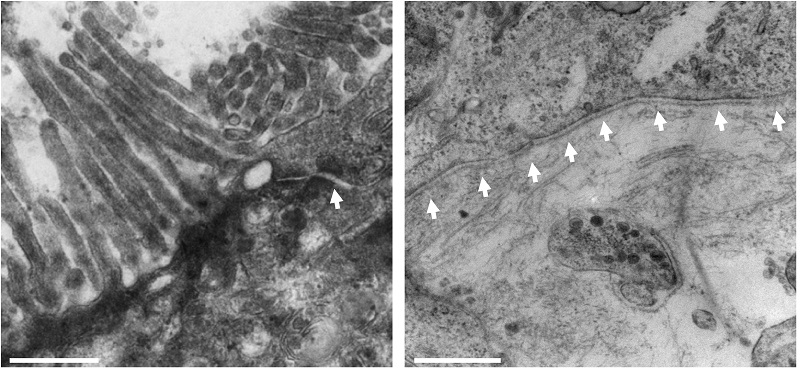
Figure 9: TEM images of the epithelium show microvilli and electro dense junction complexes, indicated by arrows (left). Basement membrane formation is also observed at the interface of caco-2 epithelial cells and CCD-18co fibroblasts (right). Scale is 500nm

REPROCELL’s contract research services use human living tissues to better predict drug safety and efficacy.
Examples of customized Alvetex intestinal models
The complexity of this in vitro intestinal epithelial model can be increased by the addition of other cell types; for example, goblet or immune cells can be added to mimic Crohn's disease and ulcerative colitis. These advanced systems can be used to study the behavior or metabolism of test articles in vitro.
Inclusion of Mucus Goblet Cells
Goblet cells create mucus in the gut, and can be added to Alvetex in the form of HT29-MTX cells. These cells retain their phenotype when cultured on HDFn, producing mucin which can be detected using PAS staining. No multilayers of these HT29-MTX cells were observed when co-cultured with fibroblasts, as would be expected in vivo.
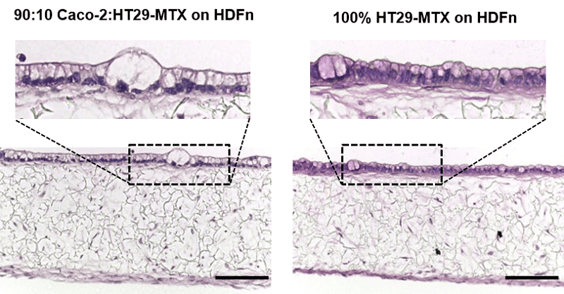
Figure 10: H&E staining of the mucosal model containing Caco-2 and HT29-MTX cells (90:10) or HT29-MTX cells only. Scale bar: 100 µm.
Modeling inflammatory bowel disease with Alvetex
Inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn's disease, affect over 10% of the world's population. In these diseases, the intestinal epithelium becomes compromised and leaky, causing immune cells to accumulate within the gut wall. By introducing immune cells into the stomal compartment of our intestinal model, we can mimic IBD in vitro (figure 11).
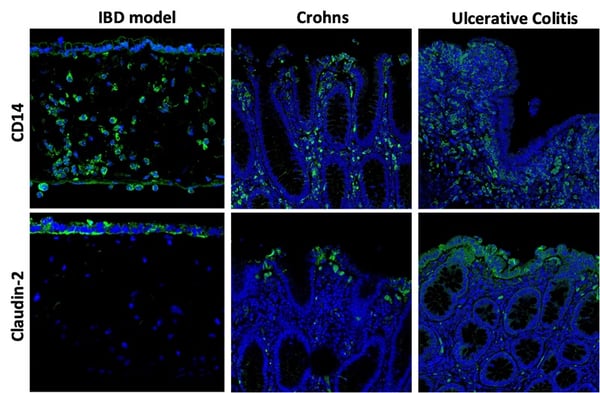
Figure 11: (Top row) CD14 staining (green) shows THP-1 cells now within the IBD model: the round, nuclei-ed cells here are within the stromal compartment beneath the epithelium. (Bottom row) Claudin-2 staining highlights how the membrane permeability is compromised by the presence of TPH-1 cells.
TEER analysis of IBD tissues
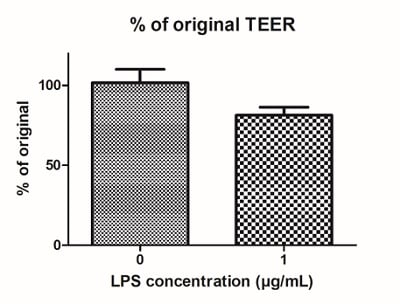
As seen from the immunocytochemistry analysis above, the presence of immune cells in this model visibly decreases membrane integrity. Membrane permeability can be further increased by stimulating the immune cells with inflammatory compounds, such as LPS and IFN-γ. As a result, the trans-epithelial resistance (TEER) was decreased in this stimulated IBD model compared with the healthy intestinal model (figure 12).
A
B
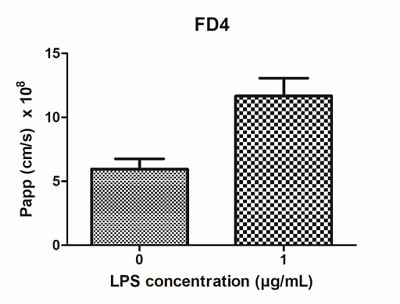
Figure 12: LPS can be used to stimulate IBD Alvetex models. Four days of LPS stimulation (1 μg/mL) results in (A) decreased TEER, and (B) increased permeability of the models.
Stratified non-keratinized epithelium
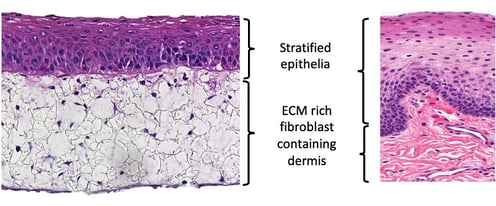
The oral, or buccal mucosa, is lined by a stratified non-keratinized epithelium that protects underlying tissues from abrasion. Our full-thickness buccal mucosa model closely resembles the real human equivalent, with an organized layer of basal cells and multiple layers of keratinocytes undergoing sequential differentiation.
Pseudostratified non-keratinized mucosa
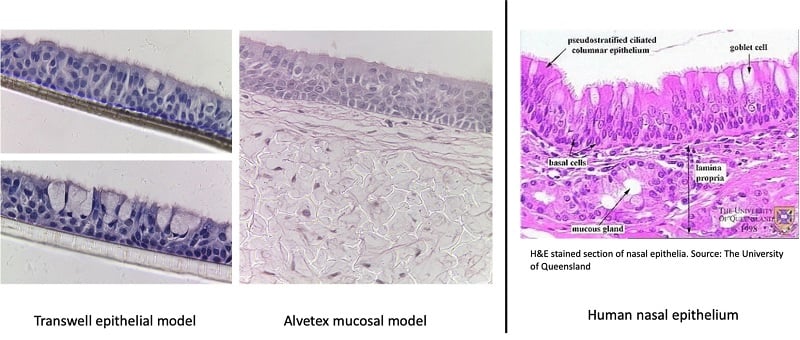
The human nasal mucosa is lined with a pseudo-stratified, ciliated epithelial layer. The Alvetex in vitro nasal mucosa model is populated with primary airway cells supported by fibroblasts, which mimics the structure of real nasal tissue. This airway model is particularly useful for studying, for example, the delivery of vaccines via nebulizers.
Using Alvetex for ADME DMPK studies
Our in vitro intestinal model can also be used for transport studies, where the metabolism or inert passage of compounds from one side of the tissue to another can be measured. This is achieved by creating intestinal membranes over one centimeter in length and mounting these structures between two chambers in an Ussing chamber system.
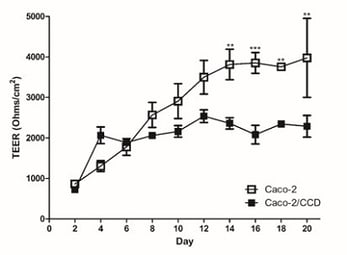
In this system, we can study electrical activity and study barrier properties by measuring TEER. Caco-2 cells are notoriously known for having abnormally high TEER values but in Alvetex, they more accurately resemble in vivo levels (figure 13).
A
B
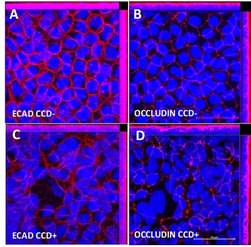
Figure 13: It is widely recognised that conventional Caco-2 Transwell models have abnormally high TEER values. (A) Human intestinal 3D models show decreased TEER values toward more physiologically relevant levels, which also correlated with (B) decreased expression of junctional proteins.
Measuring membrane permeability
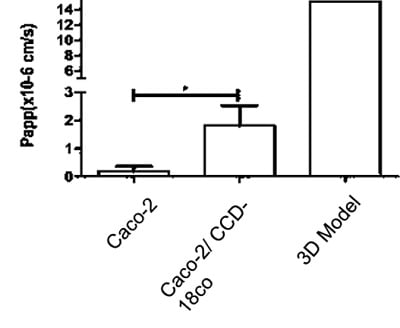
We can also study the effects of test articles on membrane permeability and how new compounds are transported across these models. Two well-known examples, methotrexate and etoposide, are shown in the graphs below (figure 14). A significant difference in transport levels is observed between the 2D and 3D systems.
A
B
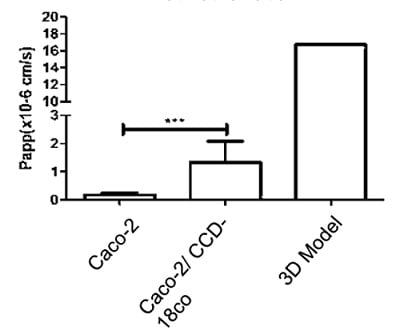
Figure 14: Comparisons of the Basal-Apical transport of (A) Methotrexate and (B) Etoposide between all three models tested (NB 3D model n=1)
Meet the inventor of Alvetex: Video Interview with Prof Stefan Przyborski
Establish translational 3D in vitro models
A number of translational human tissue models have been created using Alvetex. Our aim is to recreate the anatomy and physiology of real tissues, via careful validation and characterization, to develop bespoke assays in partnerships with our clients. If you are interested in developing a customized in vitro assay for your research, please contact our tissue engineering experts.

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.