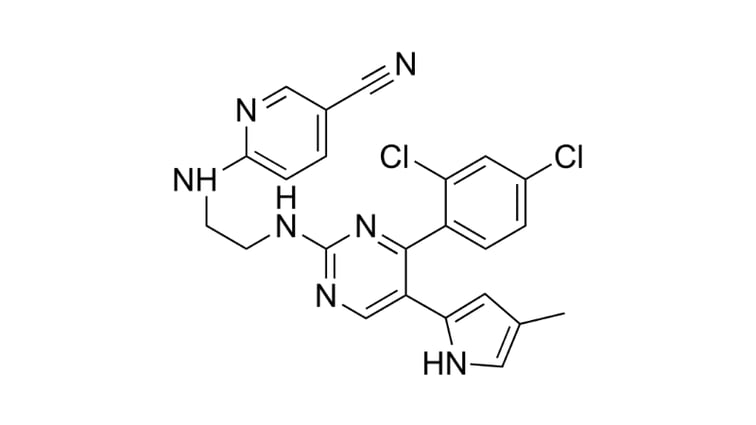
StemFit®: Mesenchymal Stem Cell therapy for liver cirrhosis [INTERVIEW]
In 2003, the worlds’ first liver regeneration therapy for cirrhosis underwent clinical trial1. In this study, 400ml of bone marrow fluid was collected from a donor under general anesthesia, transformed into Mesenchymal stem cells (MSC) and then re-infused into the same patient. Following further regulatory testing, this advanced medical treatment for liver cirrhosis was approved for use clinically.
Yet despite approval, this therapeutic was not without faults. For one, the collection and infusion of large volumes of bone marrow is a highly invasive procedure for patients - even under general anesthetic. Researchers at the Yamaguchi University Graduate School of Medicine are therefore aiming to develop a similar therapeutic using a smaller volume of bone marrow fluid. This research group, led by Professor Isao Sakaida and Associate Professor Taro Takami, is currently preparing for the clinical trial of this “self-contained cirrhosis regeneration therapy”.
Ajinomoto has been working with Associate Professor Taro Takami to develop the perfect cell culture medium for this therapeutic. The final product (StemFit® for MSC) is free of hazardous, animal-derived products, and is being used in the upcoming trial. In the following interview, Associate Professor Takami discusses his research and what his group is planning for the future.
Associate Professor, Taro Takami
Yamaguchi University Graduate School of Medicine
How is this regenerative therapeutic generated?
Firstly, MSCs are hand-cultured from the patient’s bone marrow fluid at our regenerative cell therapy center. A culture medium containing 10% fetal bovine serum (FBS) is then used to expand the MSCs in a cell culture isolator. MSCs that pass characterization tests are infused via the hepatic artery of the patient via an angiographic procedure.
Figure 1: Microscopic image of bone marrow derived MSCs cultured with StemFit For MSCs.
What has your clinical work revealed about this novel therapeutic so far?
Two subjects have already been treated in this clinical study, and no adverse events were observed during cell harvesting, culture, or infusion. A key benefit to using our therapeutic is that only 60ml of bone marrow fluid is required, rather than 400ml.
As clinical efficacy has been confirmed, we are preparing for a larger clinical trial in the second half of this year. To proceed, we plan to submit a clinical trial application to the IRB of Yamaguchi University; when this is approved, we will submit the clinical trial notification.
All our clinical research is being conducted under the Regenerative Medicine Safety Assurance Act, and the next clinical trial is being launched following PMDA consultations attended earlier this year. Here, we obtained face-to-face advice regarding standards for biological materials, quality, nonclinical safety test data, and clinical trial plans.
What were the most important considerations when planning this research?
To benefit patients, we wanted to use a lower quantity of bone marrow fluid. However, a lower volume of starting material had the potential to reduce treatment effectiveness; it was, therefore, necessary for our researchers to:
- Deliver our therapeutic to the liver efficiently and reliably: To reliably deliver our therapeutic to the liver, we changed the infusion site from the peripheral circulation to the hepatic artery. This successfully improved treatment efficacy and liver function, as confirmed in animal models.
- Produce as many high-quality cells as possible: We subsequently improved cell quality by producing MSCs with a lower passage number. This was eventually achieved by using a cell culture medium containing gamma-sterilized FBS, and StemFit for MSC which our group developed in collaboration with Ajinomoto.
How did the regulators support your research?
The PMDA advised us on all the legal compliance and safety of the raw materials and non-cellular components used in our research, which we are extremely grateful for. They suggested performing tumorigenicity and toxicity testing in the same non-clinical model, which saved our group both time and money.
Although our clinical trial was a single-dose infusion, the PMDA advised that we might need to consider repeated infusion in the future. If we do change the treatment schedule to repeated infusion, we would switch from FBS to a chemically defined medium, like StemFit for MSCs, to reduce the risk of allergic reaction.
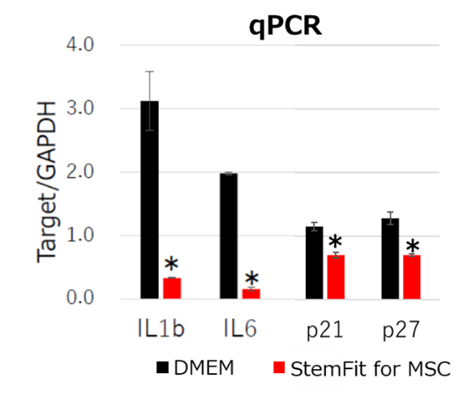
Figure 2: qPCR analysis of bone marrow derived MSCs. MSCs cultured with StemFit For MSC showed lower inflammatory gene expressions (IL1b, IL6) and aging marker gene expressions (p21, p27) compared to DMEM containing 10% FBS. * p-value <0.05
What were your quality control measures for producing MSCs?
When these MSCs were passaged for a long period of time, we noticed a reduction in anti-inflammatory activity and cell quality. Cell passage number was reduced by growing our cells using StemFit for MSC, which maintained their anti-inflammatory cytokine expression and senescence markers. We confirmed MSC quality via sterility, mycoplasma, viral, and endotoxin testing. We also used morphological analysis, cell viability, and the expression of specific surface markers, such as TSG62, as markers of MSC quality.
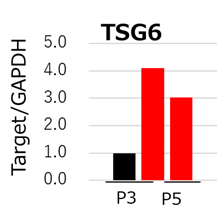
Figure 3: qPCR analysis of TSG6. MSCs cultured with StemFit For MSC (red) maintained higher TSG6 gene expression compared to DMEM containing 10% FBS (black). MSCs expressing high levels of TSG6 are shown to treat liver cirrhosis more effectively than those that do not2.
Why have you decided to avoid using FBS enriched culture media?
In our department, we try to incorporate clinically approved products from the initial research stage - even if they are costly. For us, it is important to decide which products will be used clinically as early as possible to avoid the risks caused by switching later. For example, when using proteins, recombinant varieties are recommended because it is easier to evaluate their components and manufacturing processes. So, the use of FBS for clinical work should be avoided due to variation between lots which can compromise experimental safety and reproducibility.
Why did you switch to StemFit for MSC?
Using StemFit for MSC, we were able to efficiently grow clinical cells and maintain MSCs functions at a high level, which avoids the risks associated with FBS supplemented medium. Working with Ajinomoto allowed us to explore the factors important for making this switch; for example, the FBS supplemented medium contained trypsin inactivating factor, but StemFit for MSCs did not. So, with StemFit for MSC, we had to ensure any residual trypsin was removed so that it was not carried into the fresh culture medium during passaging.
What are your plans for future research?
For over a decade, our group has worked relentlessly on the development of this liver regeneration therapy. We hope that this product will receive regulatory approval as soon as possible so that it can be accessed by the patients who need it. In future work, we would like to use our knowledge of liver cirrhosis to develop treatments for fibrosis in other organs.
References
- Terai et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion. Stem Cells 24:10 (2006)
- Miyaji et al. Bone marrow-derived humoral factors suppress oxidative phosphorylation, upregulate TSG-6, and improve therapeutic effects on liver injury of mesenchymal stem cells. Journal of Clinical Biochemistry and Nutrition 66:3 (2020)

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.