Protocol for immunocytochemistry of stem cells
Planning an immunocytochemistry (ICC) project can be tricky. It requires careful selection of compatible antibodies, fluorochromes, and blocking reagents, plus optimization of results if there is a high background signal. In this article, our scientists describe how ICC can be used to identify markers of pluripotency and provide troubleshooting tips to optimize your analysis. Download our ICC starter pack to access our double and single staining protocols for free.
REPROCELL offers a complete portfolio of services for the reprogramming and differentiation of high-quality iPSCs.
Planning your immunocytochemistry analysis
Before beginning your analysis, you must determine which pluripotency markers to visualize. Markers of pluripotency are found either on the surface or inside of the cell - some of the most popular markers and their cellular locations are shown in the table below:
| Marker | Intracellular or extracellular? |
| OCT4 | Intracellular |
| NANOG | Intracellular |
| SOX2 | Intracellular |
| TRA-1-60 | Surface |
| TRA-1-81 | Surface |
If you want to stain two markers simultaneously (double staining) ensure that one marker is intracellular and the other is extracellular, as this will make them easier to tell apart under the microscope. You should also ensure that the primary antibodies you select are from different species (e.g. rabbit and mouse) and that the secondary antibodies you choose are conjugated to differently colored fluorophores (e.g. GFP and Cyan).
Using a spreadsheet to match antibodies and markers can make this planning process easier. We have created an Excell sheet in our ICC Starter Pack which includes a tried-and-tested example of antibodies you can stain together. In this pack, we have also included an experimental checklist to ensure you have covered all the necessary steps before beginning your experiment, and both single and double staining ICC protocols, dependent on your analytical needs. You can find a range of antibodies and staining kits on the REPROCELL website.
Immunocytochemistry protocol for cultured cells
Once you have determined what antibodies you are going to use, you can begin ordering experimental reagents. Make sure to risk assess your project and complete the necessary COSHH forms before beginning any laboratory work. You could use a tool like this one provided by the HSE to get started.1
Also, make sure that you have all the necessary laboratory equipment available, and that you include negative and positive controls in your analysis. When you are ready to execute your experiment, follow our 15-step protocol below.
Stage 1: Adding Primary Antibodies
- Seed and culture cells in a 24-well plate until ready for ICC analysis. We recommend 30-40% confluency for iPSCs.
Note: Any tissue-culture-treated 24-well plate should work. Internally, we use the Falcon ones from Corning (Cat #353047). In addition, the cell-substrate iMatrix 511 should be used as a pre-coating or as an add-on at the time of passage. - Wash each well 3 times with 0.5 ml of room temperature PBS.
- Fix each well by adding 0.25 ml of 4% paraformaldehyde in PBS and incubating for 20 minutes at room temperature.
- Aspirate the 4% paraformaldehyde and then wash each well 3 times with 0.5 ml of PBS for 5 minutes with gentle agitation. Fixed cells may be stored in 1mL of PBS at 4°C overnight.
Note: If visualizing intracellular markers perform this step. Otherwise, proceed to step seven.
- Aspirate the PBS and add 150 μL 0.2% Triton-X-PBS to each well. Incubate for 10 minutes at room temperature.
- Aspirate the 0.2% Triton-X-PBS and wash the wells with 0.25 mL PBS per well.
- Add 0.5 ml blocking buffer per well and incubate at room temperature for 1 hour.
- Dilute the primary antibodies in blocking buffer according to the manufacturer’s instructions.
- Aspirate the blocking buffer and add 250 µl of each diluted primary antibody to each well. Incubate at 4°C for at least one hour. We recommend incubating overnight.
Stage 2: Adding Secondary Antibodies
- After the incubation time wash each well 3 times with 0.5 ml of PBS for 10 minutes with gentle agitation.
Note: If using a conjugated antibody, skip steps eleven through fourteen and go directly to step fifteen. If using a purified primary antibody, continue to step eleven.
- Dilute the fluorophore-conjugated secondary antibodies in blocking buffer, according to the manufacturer’s instructions, and add 250 µL of each antibody to each well.
- Incubate at room temperature for one hour, protecting the plate from light.
Stage 3: Visualizing cell nuclei with DAPI
- Following incubation, wash each well 3 times with 0.5 ml of PBS for 10 minutes with gentle agitation.
- Prepare a 2 µg/ml working solution of DAPI by diluting with PBS. Add to each well and incubate for 10 minutes at room temperature.
- Wash each well once with 0.5 ml of PBS for 5 minutes with gentle agitation.
- Aspirate any remaining PBS and add 1 to 2 drops of mounting medium to each well to stain the nuclei and preserve the samples for fluorescence microscopy imaging. Alternatively, prepare a 0.2 µg/mL DAPI solution to stain the nuclei and visualize the cells.
Examples of immunocytochemistry data
| A | 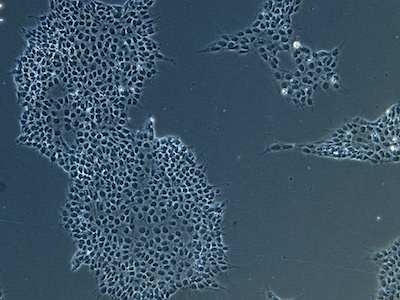 |
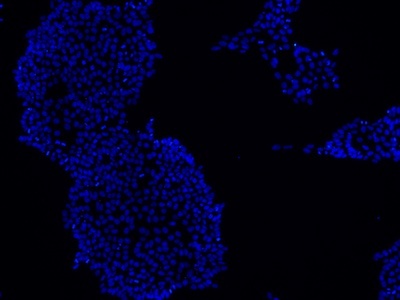 |
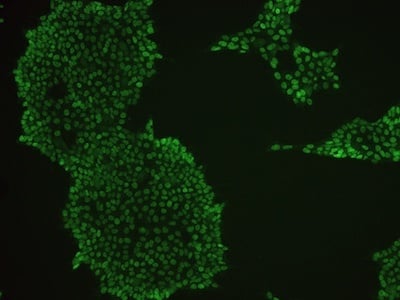 |
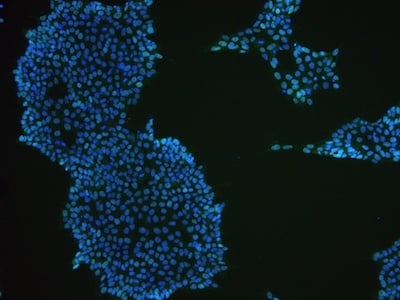 |
| B | 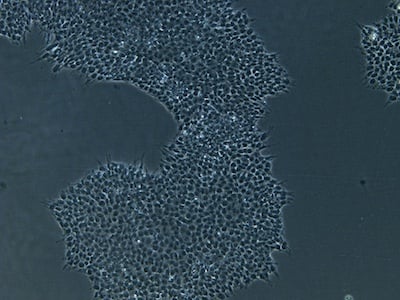 |
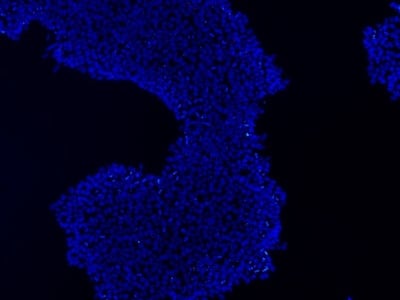 |
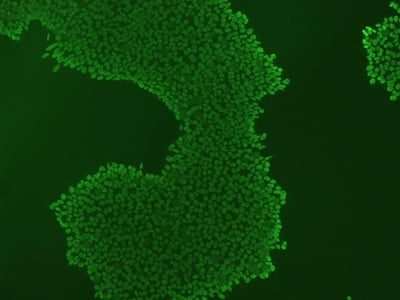 |
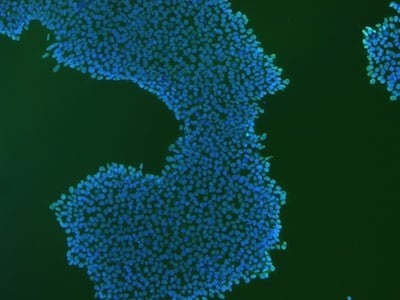 |
| A | 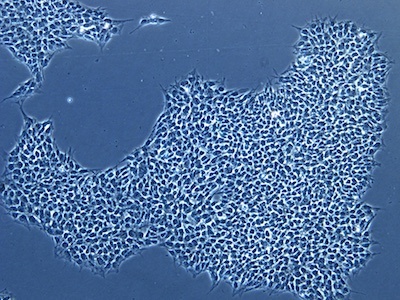 |
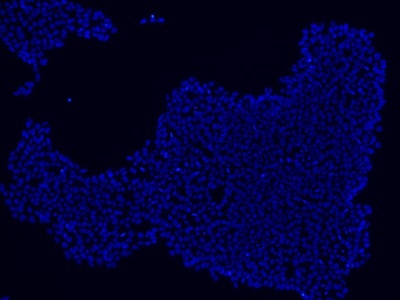 |
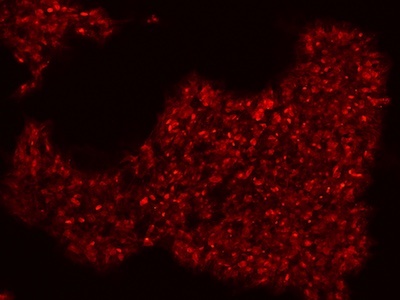 |
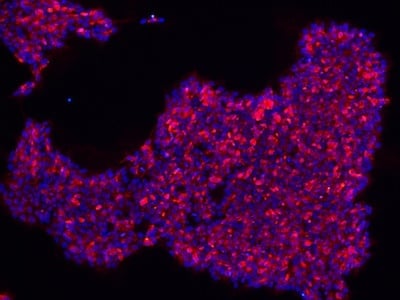 |
| B | 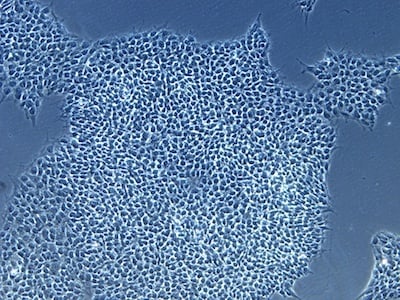 |
 |
 |
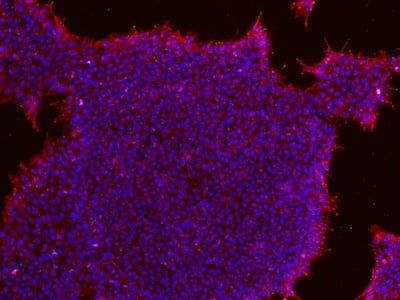 |
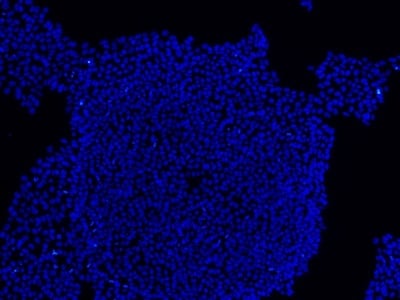
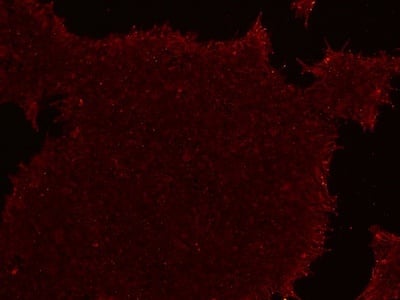
Figure 2: Characterization of fibroblast-derived iPSCs using single staining. These iPSCs were characterized by their expression of cell surface pluripotency markers TRA-1-60 (A) and SSEA-4 (B). First column: Phase contrast image of iPSCs; Second column: Visualization of cell nuclei (blue: DAPI); Third column: Visualization of cell surface markers (red: Alexa Fluor 594); Fourth column: Merge of the second and third panels. All images were taken at 10× magnification.
Optimize your ICC: High background signal
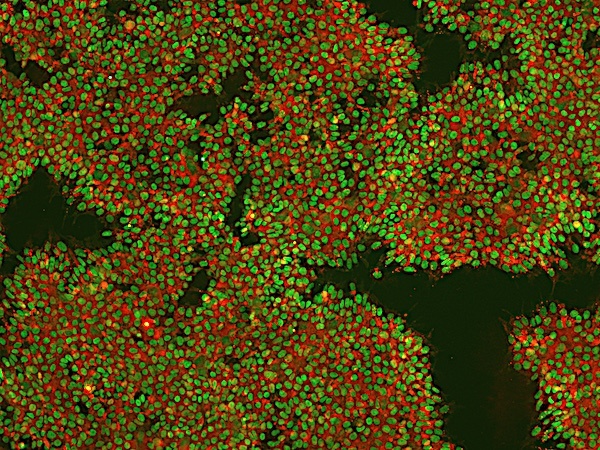
If you are experiencing a high background signal, it is likely that some of the antibodies are binding to non-specific sites. For example, the plate wells, debris, or proteins that you are not targeting. There are a few steps you can take to reduce a high background signal:
-
Increase the number of washes you perform between incubations or increase the length of these washes.
-
Increase the percentage of serum in your blocking buffer. For example, if you were using 10% animal-free blocker, increase this to 15%
-
Reduce the concentration of antibody used during incubation For example, if you were using 0.1% antibody diluted in blocking buffer, try 0.05%.
-
If all else fails, try switching from a polyclonal antibody to a monoclonal antibody to reduce cross-reactivity. We sell a number of monoclonal antibodies, which you can view on our website.
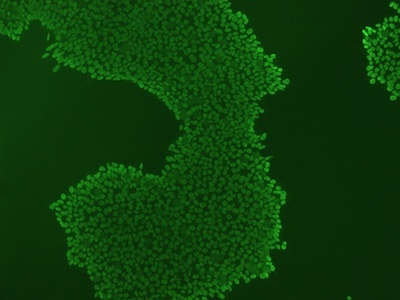
Figure 3: An example of an ICC image with high background signal. High background can be reduced by increasing the concentration of blocking buffer, increasing antibody concentration, or switching to monoclonal antibodies.
Optimize your ICC: Weak immunofluorescence signal
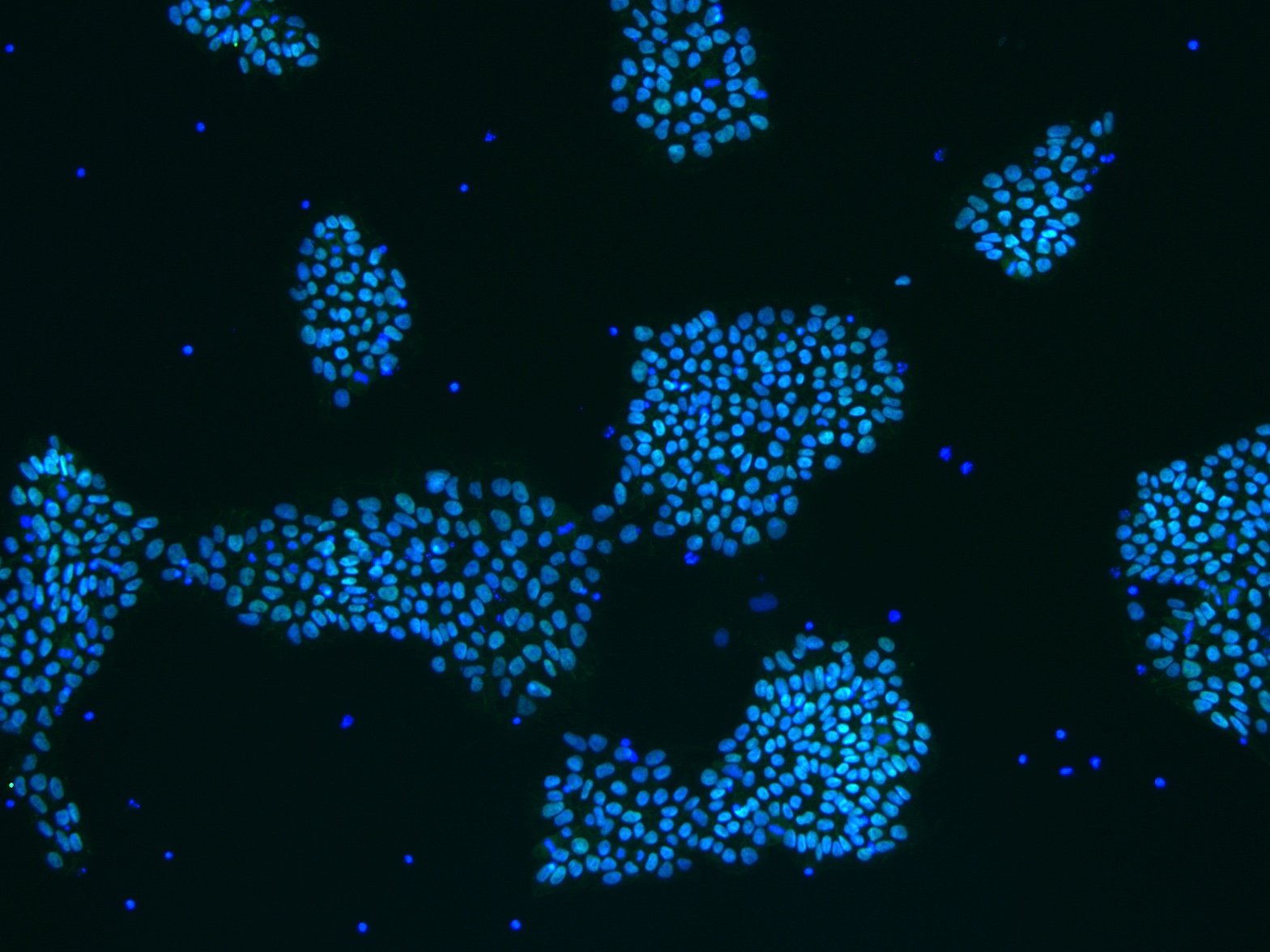
A weak immunofluorescence signal can be due to several factors, including antibody concentration, blocking buffer concentration, and errors in experimental technique. Below we have listed some of the most common causes.
- Reduce the number of washes you perform between incubation or decrease the length of these washes.
- Reduce the percentage of serum in your blocking buffer, or switch to an animal-free blocking agent.
- Increase the concentration of antibodies used during incubation
e.g. if you were using 0.1% antibody diluted in blocking buffer, try increasing this to 0.2%. - If you are staining for an intracellular marker, ensure that you have permeabilized the cell membrane using Triton X-100 or a similar reagent.
- Ensure that you are using the correct filter for visualizing the fluorescence signal with your microscope. If the microscope cannot view the wavelength of your chosen fluorophore, you will not be able to view the markers.
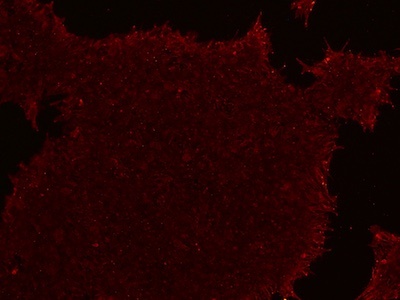
Figure 4: An example of an ICC image with a reduced fluorescence signal. The signal can be increased by increasing the antibody concentration, increasing the blocking buffer concentration, or switching to an animal-free blocking buffer.
Stem cell characterization services
As part of our contract services at REPROCELL, we offer induced pluripotent stem cell (iPSC) characterization by immunofluorescence – meaning you don’t need to spend valuable time and resources planning and optimizing your characterization project.
Visualize several pluripotency markers
We can visualize a range of intracellular and cell surface pluripotency markers including OCT4, NANOG, and SOX2. Stem cell nuclei can also be counterstained with DAPI. With our double staining service, you can visualize intracellular and cell surface markers in the same stem cell line at the same time.
Work with a dedicated study director
We will assign your project a dedicated study director who will stay in contact throughout the duration of your custom project. If you have any questions or concerns about your study, they will be happy to answer any questions that you have about the work being carried out for you.
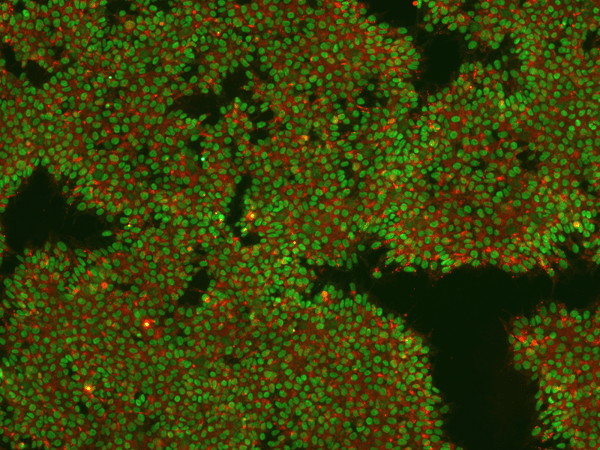
Figure 5: Image of iPSCs stained for pluripotency markers Nanog (intracellular, green) and TRA-1-60 (extracellular, red) in REPROCELL's Glasgow laboratory. Image taken at 10× magnification.
Published papers that use our antibodies
Yuancheng L et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2021.
How did you get on?
Taking on an immunofluorescence project can be daunting – especially when multiple antibodies and markers are involved. Hopefully, the information and resources we have provided will leave you feeling more confident about executing your immunofluorescence experiment. Let us know how you get on in the comments below, and follow us on LinkedIn for more stem cell articles.

REPROCELL offers a complete portfolio of services for the reprogramming and differentiation of high-quality iPSCs.
References
- HSE COSHH Essentials
- History of Immunohistochemistry in Pathobiology of Human Diseases: A Dynamic Encyclopedia of Disease Mechanisms (2014)
Note: This blog post was originally published in March 2018 but has been since updated for accuracy and clarity.

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.