Patient-derived tumor organoids recapitulate clinical treatments
The pharmaceutical industry suffers from very high rates of clinical failure, because preclinical predictions of efficacy often fail to realize a clinical benefit. In part, this can be attributed to an over-reliance on animal models of efficacy, which often do not fully predict human responses to drugs.
Two areas where very few predictive models are available is inflammatory bowel disease (IBD)—which includes ulcerative colitis and Crohn’s disease—and gastrointestinal cancer—specifically cancer of the small intestine and the colon. REPROCELL has set out to solve these challenges by developing new preclinical tests based on human tissues, which we will explore in this article.
3D patient-derived tumor organoids
Recently, researchers from The Institute of Cancer Research, London and from the Department of Medicine, The Royal Marsden NHS Trust, London, UK, demonstrated that 3D patient-derived tumor organoids can be used to efficiently screen candidate cancer treatments. In all instances, if a drug did not have an effect when tested in a patient-derived tumor organoid model, it also was ineffective when tested in the patient. On the other hand, 90% of the drugs that were effective in patient-derived tumor organoid model, were also effective when tested in patients [1]. For the first time a study unequivocally, show that organoids made in the lab can predict accurately clinical outcomes!
 |
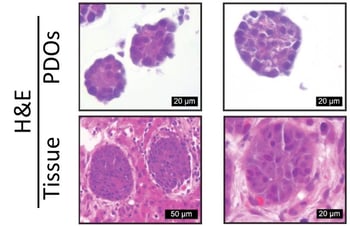 |
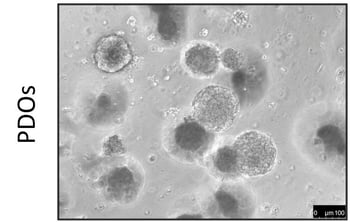
Figure 1: Phase-contrast image of patient-derived organoids (PDO) (left) and their matching patient biopsy (H&E staining; right)
3D human micro-intestine model
In addition to its predictive models using fresh IBD tissues, REPROCELL is currently evaluating the use of a highly predictive three-dimensional (3D) human micro-intestine model that can be used to test new drugs for the treatment of IBD and gastrointestinal cancers. The cornerstone of the model is three-dimensional (3D) human micro-intestines, which are obtained by isolating intestinal stem cells plus Paneth cells from the intestinal crypts of the small intestine or the colon.
Since intestinal stem cells can differentiate into all intestinal epithelial cell types, these micro-intestines are formed solely of normal, differentiated non-transformed human epithelial cells. As a consequence, these micro-intestines retain their biological relevance and thus are well suited to predict the effects of new drugs. Importantly, these micro-intestines can survive in culture for weeks to months and, potentially, can be frozen and thawed while remaining functional [2,3].
The cutting edge pre-clinical models being developed at REPROCELL are made possible by access to human tissues from a diverse collection of patient cohorts. Using these human tissues, REPROCELL is also developing a precision-cut tumor slicing methodology that retains tumor complexity and heterogeneity. An advantage of this method is that it retains the spatial arrangement of cells within the tumor, making these slices extremely valuable for the preclinical assessment of anti-cancer drugs.
References
- Vlachogiannis et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. 359 (2018)
- Sato et al. Growing self-organizing mini-guts from a single intestinal stem cells: mechanism and applications. Science. 340:6137 (2013)
- Shamir et al. Three-dimentional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 15:10 (2014)
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.
