4 reasons human tissues should be used to predict drug bioavailability

Oral administration continues to be the most common route of drug delivery. As pharmacological development costs continue to rise, ADME/DMPK researchers are keen to pursue models which can more effectively predict human bioavailability. In this post we will explore why accurate prediction of gastrointestinal (GI) permeability is important, and list four key benefits to using fresh human tissue to estimate drug permeability.

REPROCELL’s contract research services use human living tissues to better predict drug safety and efficacy.
1. Human tissue allows you to evaluate absorption across many GI regions
The composition of the intestinal membrane and luminal fluid can change depending on intestinal region [1]. For this reason, the small intestine displays increased permeability to many compounds compared with the colon, and the jejunum displays the highest rate of intestinal ion transport.
Current in vitro models fail to replicate this variation, meaning there is increased interest in more translational models, such as in vivo studies and fresh tissue assays [1]. By testing novel molecules in human tissue that is fresh and functional, transport and metabolism across the GI tract can be estimated and compared with data from animal systems or various areas of the GI tract such as the stomach or colon.
2. Human tissues avoid species differences
There is increasing knowledge that the GI tract can contribute to low oral bioavailability due to metabolism in the gut by metabolic enzymes [1]. CaCo2 cell models express many of the same transporters as the human intestine (albeit not always in physiologically relevant levels) but very low levels of CYP3A4 [1].
Animal tissues are also commonly used, as they are more complex than in vitro models and mimic human passive permeability well. However, both non-human primate and rat models show poor correlation with human oral bioavailability due to species differences in enzyme and transporter expression [2,3]. Ussing Chamber studies using fresh human tissue are therefore the only model which can help predict the oral permeability of a drug at the site of gut absorption.

3. Measure drug absorption, metabolism and transporter effects simultaneously
One disadvantage of using high capacity screening tools is that data obtained in isolation rarely predicts human in vivo drug behavior accurately [1]. A key benefit of measuring drug absorption, transporter effects, and metabolism in a single experiment is more relevant data, plus reduced cost compared with performing individual assays. In the graph below, we can see that Ussing chamber studies show good correlation between Papp and clinical drug permeability across a range of different compounds (Figure 1).
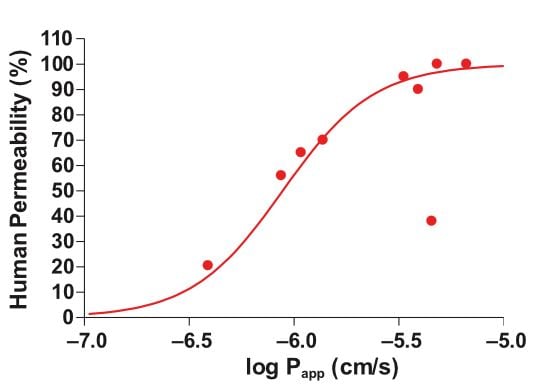
Figure 1: Relationship between drug permeability (Papp) in healthy intestine and clinical drug permeability in humans.The Papp and clinical permeability values for nine drugs (Warfarin, Methotrexate, Clonidine, Digoxin, Verapamil, Mannitol, Atenolol, Antipyrine, Sulfasalazine) were measured and then plotted on the above graph, showing good correlation between the two values.
4. Estimate GI permeability in IBD tissue
The use of whole human tissue allows researchers to estimate drug behavior in GI tissues from IBD patients. For example, if your drug is designed to treat patients with Ulcerative Colitis (UC) an Ussing Chamber can be used to test drug absorption in inflamed GI tissues from the relevant patient group. A recent Ussing study by REPROCELL showed that UC donors absorb drugs differently from healthy individuals, some demonstrating increased drug absorption and others showing decreased permeability (Figure 2).
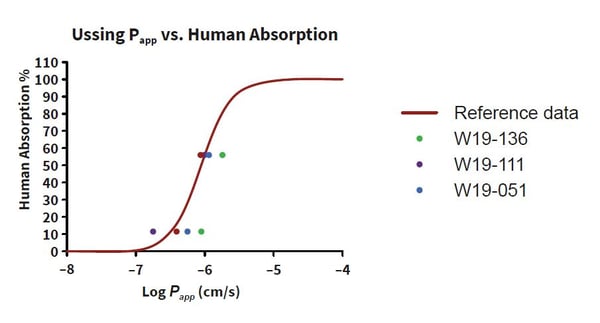
Figure 2: Relationship between permeability and human absorption in intestinal resections. Papp values were calculated as a mean of the four baths in each donor and then plotted against the reference data. The absorption profile for the healthy donor [W19-136] was comparable to the reference data, whilst data for the Ulcerative Colitis (UC) patients varied from the reference data [W19-111; W19-136].

REPROCELL’s contract research services use human living tissues to better predict drug safety and efficacy.
Predict drug permeability pre-clinically
Correct prediction of drug permeability prior to first-in-man studies can reduce clinical failure due to inadequate efficacy. The ability to more accurately predict the effective dose of your drug increases confidence heading into clinical trial and ensures you the best chance of getting your drug to market. As fresh human tissue assays are the closest method to testing in the clinic, you can be assured of the most translational preclinical data from Ussing studies using fresh human tissues.
REPROCELL’s customized Ussing studies represent the only commercially available method to estimate drug behavior in whole, fresh human tissues. To learn more about the benefits of fresh human tissue testing, watch our video by Dr David Bunton below or contact one of our experts.
References
- Sjöberg et al. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. European Journal of Pharamacological Science 48 (2013)
- Lennernäs. Animal data: The contributions of the Ussing Chamber and perfusion systems to predicting human oral drug delivery in vivo Advanced Drug Delivery Reviews 59:11 (2007)
- Chiou et al. Comparison of Oral Absorption and Bioavailability of Drugs Between Monkey and Human Pharmaceutical Research 19 (2002)

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.