4 ways stem cells can be used in medical treatments

Stem cell therapeutics promise to solve many medical and scientific problems. However, the introduction of these therapies is limited by our lack of knowledge about their effects in humans. In this post, we will review four future applications of clinical-grade induced pluripotent stem cells (iPSCs) in medicine and research – including their use in drug discovery and regenerative therapeutics.

Revolutionizing the way medicines are developed and selected for patients.
What does "Clinical Grade" mean?
Clinical Grade iPSCs are stem cells generated under Good Manufacturing Practice (GMP). Compared with their embryonic and progenitor counterparts, clinical grade iPSCs carry a reduced risk for transplant rejection [1,2] because they carry the same genetic material as the donor they were derived from. These cells can be generated from a wide range of tissues – including blood, skin and urine – and possess compatible pluripotency to embryonic stem cells if made with high quality reprogramming technology [2,3].
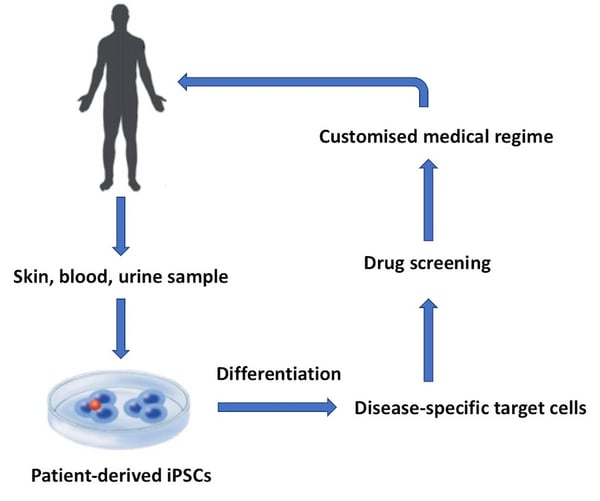
Figure 1: Human iPSCs can be used for a range of applications in drug discovery and medicine. This flow diagram shows a basic workflow of some of the key applications for clinical iPSCs.
1. Human Blood transfusion
Artificial human blood, or cultured red blood cells (cRBCs), may be the first clinical application of regenerative medicine. Already, scientists across Japan, USA, Australia and England have been studying artificial blood production with the aim of creating universal RBCs for transfusion [4, 5].
There are several financial and medical benefits to the introduction of cRBCs for blood transfusion, including:
- Decreased risk of infection
- Reduced risk of alloimmunization
- Relief from potential blood shortages
- Reduction in logistical constraints of transfusion [6]
Introduction of this technology means that patients with hereditary blood disorders or rare blood types would benefit from an unlimited stock of healthy blood for transfusion [5].
2. Replacing tissues and organs
One person dies every minute from a disease which could be treated by tissue or organ replacement [6] and current transplantation systems suffer from limited donor supply [2]. Due to their decreased risk of transplant rejection, clinical iPSCs have the potential to regenerate lost tissues and whole organs for transplantation, eliminating the need for human organ donors [2].
[READ] 4 Ted Talks discussing the future of regenerative medicine →
The potential benefits of this technology are huge, however, we need to broaden our understanding of transplant techniques, iPSC behavior, and tissue regeneration before the artificial growth of whole human organs will be possible [2, 5]. You can find out more about the future of whole organ transplantation in the TED Talk below.
3. Cellular therapies
The most exciting potential application of clinical iPSCs is their ability to cure inherited neurological disorders in adults such as Parkinson’s Disease and Spinocerebellar Ataxia. Already, several cell therapies have been approved by the FDA and are commercially available for clinical use such as Carticel, a cellular therapy to treat articular cartilage defects [2].
[READ] Stem cells offer hope for sufferers of Spinocerebellar Ataxia →
None of the currently approved regenerative therapies use iPSCs, yet these cells are attractive an source for stem cell therapeutics [2]. Mesenchymal stem cells (MSCs) are being explored as a treatment for multiple sclerosis (MS), brain trauma, and cardiac degeneration [2]. So far, the observed effects have been positive, meaning there is great interest in identifying iPSC populations for therapeutic use [2].
| Therapeutic | Company | Approved in | Derived from | Used to treat |
|---|---|---|---|---|
| Prochymal | Mesoblast | Canada | Bone marrow | Graft versus host disease |
| Alofisel | Takeda Pharma | Europe, Switzerland | Adipose tissue | Perianal fistula |
| Stempeucel | Stempeutics Research | India | Bone marrow | Critical limb ischemia |
| TemCell HS | JCR Pharmaceuticals | Japan | MSCs | Acute graft versus host disease |
| Stemirac | Japan | MSCs | Spinal cord injury | |
| Prochymal | Osiris Therapeutics | New Zealand | Acute graft versus host disease | |
| Neuronata-R | CoreStem | Korea | Bone marrow | ALS / Lou Gehrig's disease |
| Cupistem | Anterogen | Korea | Adipose tissue | Crohn's fistula |
| CARTISTEM | Medipost | Korea | Umbilical cord blood | Knee articular cartilage defects |
| Cellgram-AMI | PharmiCell | Korea | Bone marrow | Acute myocardial infarction |
| Queencell | Anterogen | Korea | Adipose tissue | Subcutaneous tissue defects |
Table 1. List of currently approved stem cell therapeutics. List originally compiled in this article.

Revolutionizing the way medicines are developed and selected for patients.
4. Drug discovery and precision medicine
Already, iPSCs have been successfully used to study the phenotype of several diseases including Huntington’s, Alzheimer’s, and Parkinson’s Disease [6]. Researchers around the globe are using iPSC-derived models to reduce drug attrition rates between preclinical testing and clinical trial, particularly where living patient samples are impossible to access, such as the nervous and cardiac systems.
Examples of commercially available iPSC-derived models include:
- SynFire® Induced GABAergic and Glutamatergic Neurons (NeuCyte)
These neurons can be used to assess the neurotoxicity of compounds and disorders of excitatory and inhibitory brain circuits. Read more about how SynFire® neurons can benefit your research in this article. - StemRNA™ Neuro (REPROCELL)
CNS neurons derived from iPSCs which can be used to study drug efficacy and safety in vitro.
With enough experience and knowledge of iPSC-differentiation, any cell in the body could be generated from iPSCs making them a dynamic solution to the current lack of translational disease models and “one-size-fits-all” approach to drug prescribing. Researchers have already expanded their differentiation capabilities to include sensory neurons and glial-restricted progenitors, with the aim of differentiating even more neuronal cell-types in the future.
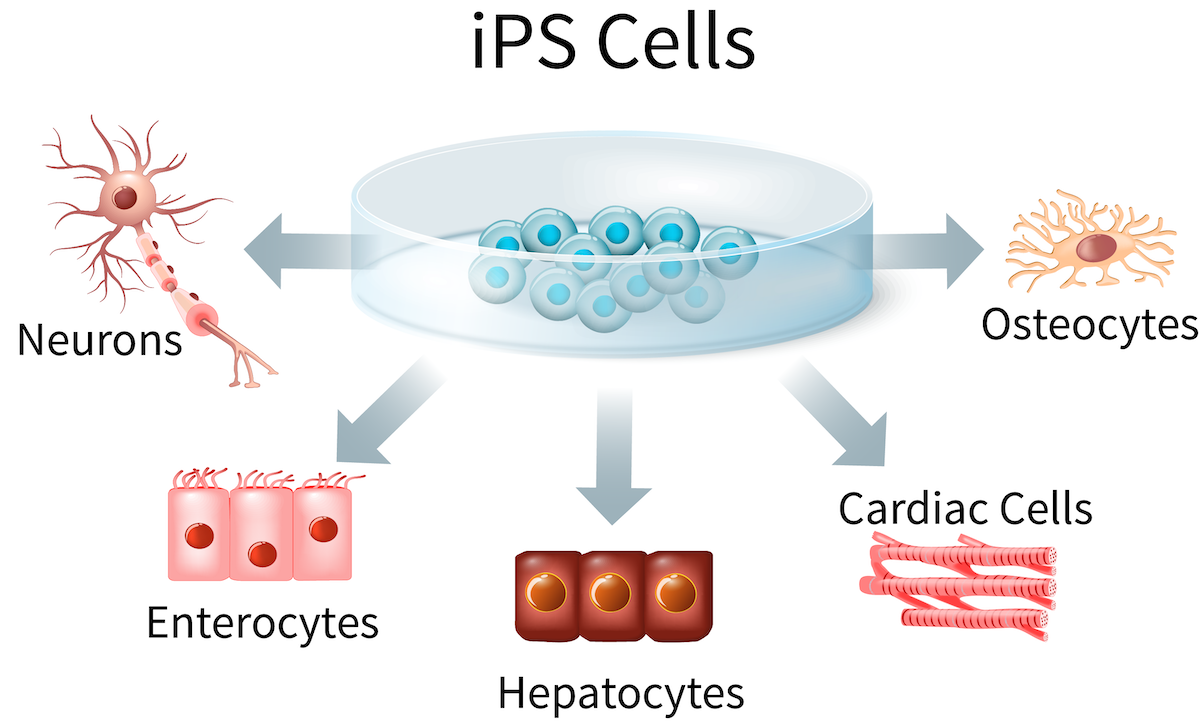
Figure 2: Induced pluripotent stem cells (iPSCs) can be used to generate a wide range of tissue types, including cells from the liver, intestine, heart, bone and nervous system.
Learn more about clinical iPSC technologies
In this article, we have highlighted some of the key advancements and challenges facing the introduction of stem cell therapeutics. Let us know what you think on our LinkedIn page or in the comments below.
- Cell and Gene Therapy Catapult
This organization have a range of approaches that can help tackle the challenges you might face in the development of cell and gene therapies. They will work with you to accelerate your projects, helping your cell and gene therapies to be safer, more effective, scalable and affordable.
- Centre for Regenerative Medicine
The center for regenerative medicine (CRM) in Edinburgh is positioned uniquely to translate scientific knowledge to industry and the clinic. Their research is aimed at developing new treatments for major diseases including cancer, heart disease, liver failure, diabetes, and degenerative diseases such as multiple sclerosis and Parkinson's.
- REPROCELL’s latest clinical iPSC newsletter (use the form below)
Subscribe to our exclusive newsletter and be the first to hear new developments in regenerative medicine and out stem cell services.

Revolutionizing the way medicines are developed and selected for patients.
References
- Turinetto et al. Induced Pluripotent Stem Cells: Advances in the Quest for Genetic Stability during Reprogramming Process. International Journal of Molecular Sciences 18:9 (2017)
- Mao et al. Regenerative Medicine: Current therapies and future directions. PNAS 112:47 (2015)
- Akahashi et al. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (2006)
- In vitro stem cell derived red blood cells for transfusion: Are we there yet? Yonsei Medical Journal 55:2 (2014)
- Why industrial production of red blood cells from stem cells is essential for tomorrow’s blood transfusion. Regenerative Medicine 13:6 (2018)
- Schlaeger et al. A comparison of non-integrating reprogramming methods. Nature Biotechnology 33:1 (2015)

Author
Zara Puckrin, BSc
Zara is a GCU graduate who loves minimalism, marketing, and molecular biology. You can contact her on LinkedIn.
Subscribe to receive updates from REPROCELL
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.